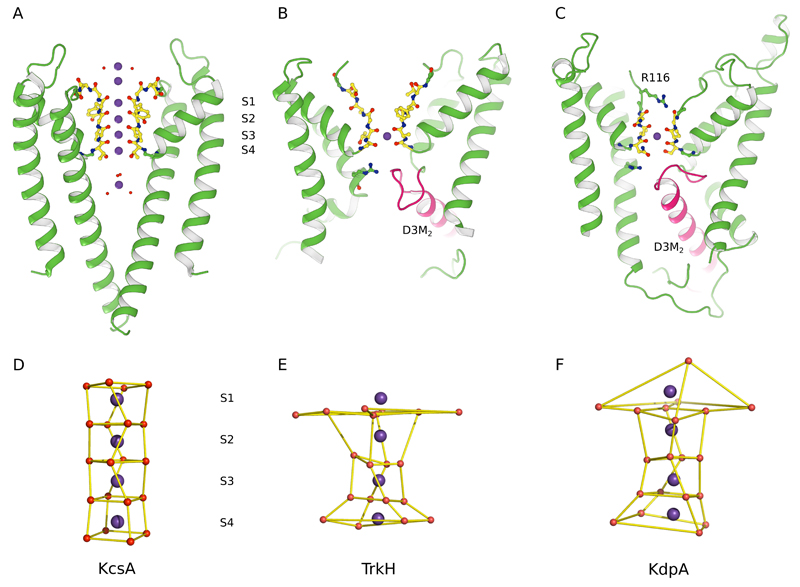
Extended Data Figure 3. Comparison of selectivity filters from a potassium channel and SKT transporters.
(A) KcsA is an archetypal K+ channel and the 2.0Å resolution structure (1K4C)17 shows four K+ sites within the selectivity filter at sites designated S1-S4. These sites are presumed to be alternatively occupied by K+ and water molecules, but differences have been masked by crystallographic averaging. Additional, partially hydrated K+ ions are seen in cytoplasmic and periplasmic vestibules at either end of the filter. (B) TrkH is more closely related to KdpA and displays the gating loop at the cytoplasmic end of the selectivity filter (pink). The crystal structure (3PJZ)11 shows a single K+ ion in the S3 position and the filter is considerably more disordered toward its periplasmic end, perhaps accounting for the lack of further ions. (C) KdpA has intermediate order in the selectivity filter. A K+ ion occupies the S3 site and the side chain of Arg116, which was been substituted for Gln in our construct, appears to occupy the S1 site. Like TrkH, a gating loop is evident from the kinked helix (pink) in the third M1PM2 repeat. (D) The cage of oxygen atoms that coordinate the K+ ions in the selectivity filter of KcsA. The strict four-fold symmetry of this channel produces a very regular geometry. (E) The analogous oxygen atoms in the TrkH structure shows considerably more distortion of this cage and complete absence of coordination at the S1 sites. (F) The coordination cage for KdpA has intermediate order with reasonable evidence for sites at S2-S4.