Fig. 1.
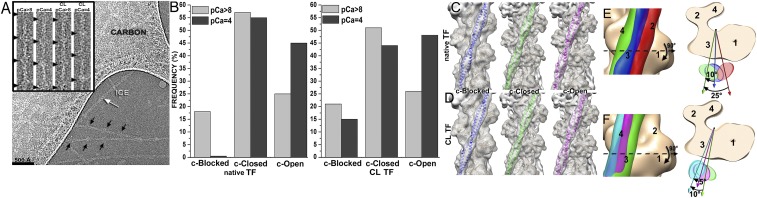
Three-dimensional reconstruction of frozen hydrated native and cross-linked cardiac TFs. (A) Electron micrograph of frozen hydrated cardiac TFs (pCa = 4) shows that some filaments possess well-defined Tn densities (black arrows), whereas others do not (white arrow). (Inset) Examples of segments of native and cross-linked (CL) TFs at low (pCa > 8) and high (pCa = 4) Ca2+ used for image analysis. Tn complexes are marked with black arrowheads. (B) Pseudoatomic models of the canonical-blocked (14), apo (15), and myosin (17) structural states of Tpm were used as reference structures in cross-correlation sorting of native and CL TFs at low (pCa > 8) and high (pCa = 4) Ca2+. (C and D) Three-dimensional reconstructions of native (C) and CL (D) cardiac TFs possessing Tpm in the c-blocked (blue ribbons), c-closed (green ribbons), or c-open (magenta ribbons) structural states. Density maps are shown as transparent gray surfaces, whereas actin subunits are tan ribbons. (E) Position of Tpm in the c-blocked state (blue surface) is compared with the previously observed canonical-blocked (14) (red surface) and apo (15) (green surface) positions of Tpm on F-actin. The top view shows an ∼10° swing of Tpm in the c-blocked state from its apo state. (F) Tpm position in the c-open state (magenta surface) is compared with the previously defined apo (15) (green surface) and myosin (17) (cyan surface) positions on F-actin. The top view shows that the c-open state is located between the apo and the myosin positions of Tpm on F-actin.