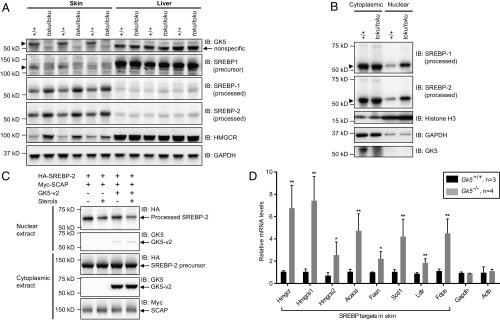
Fig. 5.
GK5 deficiency promotes SREBP processing and increases the nuclear-localized transcriptionally active forms. (A) Immunoblot analysis of SREBP-1, SREBP-2, and their target HMGCR in the skin and liver of homozygous toku and wild-type mice. GAPDH is shown as the loading control. (B) Immunoblot analysis of SREBP-1, SREBP-2, Histone H3 (nuclear marker), GAPDH (cytoplasmic marker), and GK5 in cytoplasmic and nuclear extracts from wild-type and toku homozygote skin. (C) HEK293T cells stably expressing 3xHA-tagged SREBP-2 were transfected with Myc-tagged SCAP with or without GK5-v2. Twelve hours after transfection, cells were switched to lipoprotein-deficient medium containing 50 µM mevastatin, 50 µM mevalonate, and 1% (wt/vol) hydroxypropyl-β-cyclodextrin. After incubation for 1 h, the cells were left untreated or treated with 1 µg/mL 25-hydroxycholesterol plus 10 µg/mL cholesterol (sterols). After incubation for 8 h, cytoplasmic and nuclear extracts were prepared and immunoblotted with antibodies against HA, Myc, and GK5. (D) Expression levels in the skin of SREBP targets Hmgcs1 (HMG-CoA synthase 1), Hmgcs2 (HMG-CoA synthase 2), Acaca (acetyl-CoA carboxylase α), Fasn (fatty acid synthase), Scd1 (stearoyl-CoA desaturase 1), Ldlr (low-density lipoprotein receptor), and Fdps (farnesyl diphosphate synthetase) were measured in wild-type (n = 3) and Gk5 knockout littermates (n = 4). Relative mRNA levels of each are graphed. *P < 0.05; **P < 0.01.