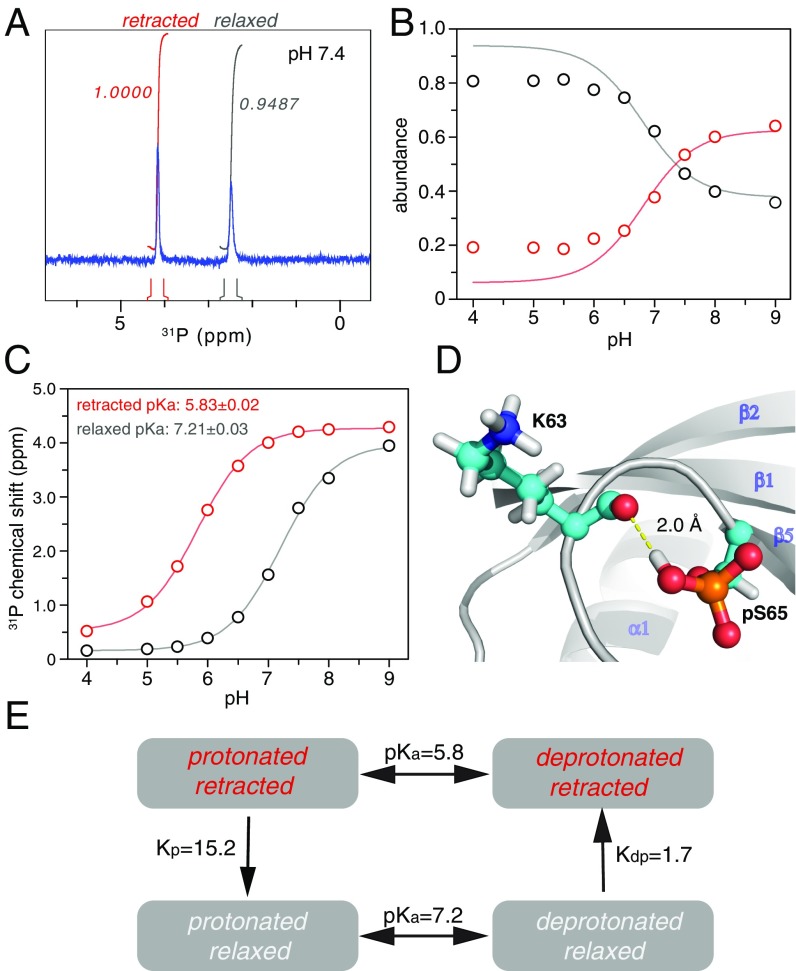
Fig. 3.
pUb exists in equilibrium among four chemical species. (A) Two 31P peaks can be observed with about the same integrated peak volumes, which can be assigned to the pS65 phosphoryl group in the retracted state and relaxed state. (B) The relative abundance of the two conformational states, quantitated by 31P peak integrals, changes in response to pH. (C) The 31P chemical-shift value changes in response to pH. The pKa values can be fitted for the retracted state and relaxed state. (D) The unusually high pKa value for the relaxed state can be attributed to a hydrogen bond between the protonated pS65 phosphoryl group and the backbone carbonyl of K63. (E) Illustration of the coupled equilibriums of pUb, with the equilibrium constants denoted. A slight decrease in pH leads to the enrichment of protonated/relaxed species of pUb, whereas a slight increase in pH leads to the enrichment of deprotonated/retracted species of pUb.