Abstract
This study aimed to evaluate the effects of black pepper petroleum extract (BPPE) on pathogenic bacteria. The extraction from black pepper showed intense antimicrobial activity against the Gram-positive Listeria monocytogenes ATCC 19115 and the Gram-negative bacteria Salmonella typhimurium ATCC 14028. The minimum inhibitory concentrations of BPPE against L. monocytogenes and S. typhimurium were 0.625 and 1.25 mg/ml, respectively. Detection of Alkaline phosphatase outside the cell revealed that BPPE treatment destroyed the cell wall integrity. BPPE also altered the membrane integrity, thereby causing leaching of 260 and 280 nm UV-absorbing materials into the medium, particularly, nucleic acids and proteins. Propidium iodide infiltration experiments also indicated that BPPE treatment altered the permeability of bacterial cell membrane. Moreover, Na+/K+-ATPase activity was inhibited by BPPE. And the results of scanning electron microscopy showed that BPPE treatment damaged the morphology of the tested bacteria. These results indicated that BPPE could destroy cell wall integrity, alter the permeability of cell membrane, and inhibit the activity of intracellular enzyme, which could kill bacteria.
Keywords: Black pepper, Ether extract, Membrane, Salmonella typhimurium, Listeria monocytogenes
Introduction
Pepper (Piper nigrum L.) was native to India and an important spice since ancient times. Pepper was introduced in China in 1947 and has been planted mainly in Hainan province. Black pepper was well known as the “King of Spices” and has been cultivated for hundreds of years (Hu et al. 2015; Nair 2004). It was widely used as medicine, preservative, and insecticide, as well as in perfumery. Pepper possessed many medicinal properties and was used to treat vertigo, asthma, chronic indigestion, colon toxins, obesity, sinusitis, and congestion (Abou-Elkhair et al. 2014; Srinivasan 2007).
Since the introduction of antibiotics over 60 years ago, they have become the main agent used to control bacterial infections (Rajmohan et al. 2010). However, using high doses of antibiotics increased bacterial resistance. An increasing number of researchers have been investigating for new active compounds against multidrug-resistant pathogens. Plant extracts and secondary metabolites possessed antifungal, antiviral, or antimicrobial activities. The main constituents of black pepper were piperine and volatile oils (Abd El Mageed et al. 2011). High Performance Liquid Chromatography analysis revealed that piperine, piperidine, eugenol, and catechin were constituents of Piper nigrum crude extract (Jin et al. 2013). In addition, trans-caryophyllene (30.33%) and limonene (12.12%) were found in black pepper oil. By contrast, the major constituent of green pepper oil was piperine (24.42%) and limonene (18.73%) (Nikolic et al. 2015). The volatile oil of black pepper demonstrated antimicrobial activity (Dorman and Deans 2000; Liu et al. 2015). The antioxidant and the antibacterial activities of different solvent extracts of P. nigrum, piperic acid, and purified piperine were varied (Zarai et al. 2013). The extracts of black pepper efficiently inhibited the growth of Gram-positive and Gram-negative bacteria, such as Staphylococcus aureus, Bacillus cereus, and S. typhimurium (Ghori and Ahmad 2009; Karsha and Lakshmi 2010). In addition, black pepper chloroform extract influences the cell morphology, respiratory metabolism, pyruvic acid content, and ATP levels of Escherichia coli and S. aureus. The extracts of black and red pepper also inhibit the DNase activity of S. aureus (Zarringhalam et al. 2013).
The present study determined the antibacterial activity of black pepper and its mode of action on bacteria by evaluating the effects of black pepper petroleum ether extract (BPPE) on the cell wall, cell membrane permeability, and correlative enzyme activity. And this study provided a theoretical basis for the storage and processing of the meat products.
Materials and methods
Chemicals
Black pepper was purchased from Peng Tai Xing Supermarket (Haikou, China). Ethanol was obtained from Xilong Chemical Corporation (Guangdong, China). Alkaline phosphatase (ALP-ase) assay kit and Na+/K+-ATPase assay kit were acquired from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). Propidium iodide (PI) was purchased from Sigma. Other chemicals of analytical grade were obtained from Xilong Chemical Co., Ltd.
Preparation of BPPE
Air-dried black pepper (200 g) was crushed with a grinder, extracted thrice by stirring with 400 ml of 80% ethanol at room temperature for 12 h, and then leached by vacuum filter. The solvent of the combined extracts was evaporated under reduced pressure by using a rotary vacuum evaporator at 50 °C, and the remaining water was evaporated at 50 °C by a thermostat-controlled water bath. Turbidity suspensions were prepared by adding 100 ml of distilled water into the obtained dried extract. Petroleum ether (100 ml) was added thrice into the suspension in a separator funnel. Low-turbidity suspensions were subsequently transferred into another vessel. Chloroform (100 ml) was added thrice, agitated, and transferred after stratification. The suspension was then successively disposed by 100 ml of ethyl acetate and butyl alcohol. BPPE was concentrated under vacuum at 30 °C, and the black pepper chloroform extract was concentrated under vacuum at 35 °C. The black pepper ethyl acetate and butyl alcohol extract were concentrated at 35 °C and 65 °C successively. All of the extracts were stored at 4 °C for further analysis.
Bacterial strains
Gram-negative bacteria S. typhimurium (ATCC 14028) and Gram-positive bacteria L. monocytogenes (ATCC 19115) were purchased from Guangdong Microbiology Culture Center. S. typhimurium was cultured on nutrient agar at 37 °C for 24 h. L. monocytogenes was grown and maintained on slants of brain–heart infusion agar (Huankai Microbial Sci. & Tech, Co., Ltd., Guangdong, China).
Determination of minimum inhibitory concentration (MIC) of antimicrobials
The MICs of the antimicrobials were determined using the standard broth micro-dilution method with slight modifications (Zhang et al. 2016). The bacteria were incubated at 37 °C overnight, harvested through centrifugation, and subsequently re-suspended to approximately 107 cfu/ml in 0.9% sterile NaCl. Serial dilutions of BPPE were prepared to obtain the final concentrations of 40, 20, 10, 5, 2.5, 1.25, and 0.625 mg/ml in agar medium (Liu et al. 2015, 2016). The sample containing the same volume of ethanol but without extract served as the negative control group, and the sample without extract and ethanol served as the blank control group. The plates were incubated for up to 24 h before recording the MICs.
Effects of antimicrobials on bacterial cell wall
ALP-ase was an intracellular enzyme that cannot pass through the intact cell wall and thus would be detected when the cell wall is destroyed (Nomoto et al. 2016; Zappa et al. 2001). ALP-ase activity was determined by using an ALP-ase kit according to the manufacturer’s instructions (Nomoto et al. 2016). S. typhimurium and L. monocytogenes were incubated in broth, and were collected in the logarithmic phase. The bacterial suspensions were prepared by stroke-physiological saline solution. The test compound at MIC was added into the suspension and then 10 ml of bacterial suspension was centrifuged at 6000 rpm for 15 min at 4 °C. The supernatant was used to detect ALP-ase.
The integrality of cell membrane
Damage in cell membranes was determined by Propidium Iodide (PI). PI cannot penetrate cells that were bound by intact membranes (Klotz et al. 2010). Bacterial suspensions of S. typhimurium and L. monocytogenes were prepared and grown overnight in nutrient broth and brain heart infusion broth under continuous shaking at 37 °C, respectively. The test compound at MIC was added into the suspension and incubated at 37 °C for 24 h. And then the suspension was centrifuged at 8000 rpm per 10 min at 4 °C and washed twice with stroke-physiological saline solution, followed by addition of 30 µM PI and incubation in ice bath for 10 min, and the group which without the BPEE as the control. The fluorescence intensity was measured at 550 nm excitation and 628–700 nm scanning emission with a 5 nm slit width at room temperature (25 °C), and the baseline was corrected using an F-7000 fluorescence spectrophotometer (Japan) (Bunthof et al. 1999).
Measurement of release of 260 and 280 nm UV-absorbing materials
The measurement of release of 260 and 280 nm UV-absorbing materials into the supernatants was determined by an ultraviolet–visible light detector (Yi et al. 2010). Bacterial suspensions of S. typhimurium and L. monocytogenes were prepared and grown in broth overnight under continuous shaking at 37 °C, harvested, washed thrice with distilled water by centrifugation each time at 6000 rpm for 10 min at 4 °C, and then the bacterial suspension was prepared to contain 107 cfu/ml in 0.9% sterile NaCl. After incubation at 37 °C for 30 min, the test compound at MIC was added into the suspension. Aliquots of the samples were drawn at regular intervals of 15 min and centrifuged, then the absorbance of 260 and 280 nm UV-absorbing materials in the suspension were measured by Ultraviolet–visible light detector (China) (Cui et al. 2012; Tian et al. 2009).
The activity of Na+/K+-ATPase
Na+/K+-ATPase activity was determined using an enzyme kit according to the manufacturer’s instructions (Liu et al. 2015). Fresh liquid medium was added into the activated S. typhimurium and L. monocytogenes culture. S. typhimurium and L. monocytogenes were collected in the logarithmic phase. The specific MIC level decided into the suspension and the remaining portion remained untreated as a control. The suspension was incubated at 37 °C for 24 h, and 20 ml suspensions in all tubes were harvested through centrifugation and then washed twice with distilled water at the test point. The cells were re-suspended with stroke-physiological saline solution and were broken with ultrasound in ice bath for 4 min (550 W, working at 10 s intervals) by ultrasonic liquid processors. And the cell debris has been removed through centrifugation at 10,000 rpm per 10 min at 4 °C. The supernatants were collected and the protein content of the mixture was examined by xylene brilliant cyanine G250 (Kayali et al. 2011). In addition, Na+/K+-ATPase activity was measured by ATPase kit.
Investigation of structural changes via scanning electron microscopy (SEM)
The cells were incubated over night under continuous shaking at 37 °C and kept the concentration approximately 107 cfu/ml in liquid medium. Antimicrobials were added into the processed groups, achieving their specific MIC levels. The BPPE-treated, alcohol-treated, and non-treated bacterial cultures were incubated at 37 °C for 8 and 24 h, respectively. The procedure was described in the following paragraph.
The cells were collected at 6000 rpm for 10 min through centrifugation. They were washed thrice with sterile phosphate-buffered saline and then dehydrated using serial dilutions of ethanol as follows: 20, 40, 60, 80, and 100%. The specimens were prepared and pre-frozen at −40 °C for 1–2 h. The samples were placed in a freezing and deep-freezing equipment. And then the samples were gold covered through cathodic spraying. The specimens were prepared and the morphology of bacterial cells was examined under Hitachi S-3000 SEM (Japan) (Cetin-Karaca and Newman 2015; Yong et al. 2015).
Statistical analysis
All experiments were performed in triplicate. Data were analyzed by ANOVA by using Origin 9.0 and SPSS 19.0. Difference between groups was considered significant at P < 0.05.
Results and discussion
Antimicrobial activity of black pepper organic extract
The increased use of antibiotic could increase bacterial resistance and encourage the search for new active compounds against food-borne pathogens (Yang et al. 2016). This study analyzed the activity of the extracts of four species of black pepper against two standard and familiar bacterial strains.
This study showed that different organic extracts demonstrated variable antimicrobial activities against the tested strains. The MICs were showed in Table 1. The BPPE and chloroform extract demonstrated similar and high antimicrobial activity against both L. monocytogenes and S. typhimurium during the 24 h incubation. BPPE displayed the highest inhibitory activity, reaching values of 0.625 and 1.25 mg/ml against L. monocytogenes and S. typhimurium, respectively. The MIC of chloroform extract was 1.25 and 2.5 mg/ml against L. monocytogenes and S. typhimurium, respectively. The MIC of ethyl acetate extract was 20 mg/ml against both L. monocytogenes and S. typhimurium. The MIC of butyl alcohol extract was 10 and 20 mg/ml against L. monocytogenes and S. typhimurium, respectively. These results showed that BPPE exhibited the strongest inhibitory activity against the growth of microorganisms than the other three organic extracts. So the BPEE was used to further study in the morphology of L. monocytogenes and S. typhimurium. These findings were consistent with those of the Philippine Piper methanol, ethanol, and supercritical CO2 extracts, which demonstrated bactericidal activity against E. coli and P. aeruginosa (Valle Jr et al. 2016). Aqueous decoction of black pepper (P. nigrum L.) evidently inhibited 176 bacterial isolates belonging to 12 different genera of bacteria (Chaudhry and Tariq 2006).
Table 1.
The antibacterial efficacy of the antimicrobials
| Microorganism | Ether extract MIC (mg/ml) | Chloroform extract MIC (mg/ml) | Ethylacetate extract MIC (mg/ml) | Butyl alcohol extract MIC (mg/ml) |
|---|---|---|---|---|
| L. monocytogenes (ATCC 19115) | 0.625 | 1.25 | 20 | 10 |
| S typhimurium (ATCC 14028) | 1.25 | 2.5 | 20 | 20 |
The integrality of cell wall
The enzymes in bacteria promote metabolism and accelerate biochemical reactions. The bacterial cells lack energy when the intracellular enzyme activity was inhibited by antibacterial agents.
The cytoderm of microbial cells plays an important role in maintaining normal growth by isolating intracellular enzymes and macromolecular substances. The enzyme ALP-ase effused from the cell when the cell cytoderm was destroyed. The ALP-ase activity was determined with an ALP-ase kit. The activity of ALP-ase significantly increased when bacterial cells were treated with BPPE at MICs. The activities of ALP-ase in the culture of L. monocytogenes and S. typhimurium were 9.708 and 9.375 U/gprot by treating with BPPE for 4 h, respectively. After 10 h, the ALP-ase activity of L. monocytogenes was 8.167 U/gprot, compared with 1.125 U/gprot for the control group. S. typhimurium treated with BPPE exhibited similar behavior (Table 2). These phenomena showed the leaching of ALP-ase from the intracellular to the extracellular medium, mainly because the cell cytoderm was destroyed in L. monocytogenes and S. typhimurium cells.
Table 2.
The activity of ALP-ase L. monocytogenes and S. typhimurium
| Time (h) | L. monocytogenes | S. typhimurium | ||||||
|---|---|---|---|---|---|---|---|---|
| CG OD520 |
CG U/gprot |
EG OD520 |
EG U/gprot |
CG OD520 |
CG U/gprot |
EG OD520 |
EG U/gprot |
|
| 0 | 0.014 | 0.583 | 0.027 | 1.125 | 0.034 | 1.417 | 0.064 | 2.667 |
| 2 | 0.015 | 0.625 | 0.127 | 5.292 | 0.065 | 2.708 | 0.109 | 4.542 |
| 4 | 0.025 | 1.042 | 0.233 | 9.708 | 0.063 | 2.625 | 0.225 | 9.375 |
| 6 | 0.022 | 0.917 | 0.212 | 8.833 | 0.052 | 2.167 | 0.221 | 9.208 |
| 8 | 0.028 | 1.167 | 0.204 | 8.500 | 0.051 | 2.125 | 0.198 | 8.250 |
| 10 | 0.027 | 1.125 | 0.196 | 8.167 | 0.076 | 3.167 | 0.203 | 8.460 |
CG control group, EG treat group
These results were consistent with the earlier findings (Cui et al. 2015), indicating that the nutmeg oil in pork could destroy the cell wall and cell membrane of E. coli and S. aureus. The leakage of ALP-ase could degrade the main nucleic acid components, such as RNA and DNA. The protection and support role of the cell wall disappeared which resulted the cell membrane and organelles were affected. Moreover, the morphology of bacterial cells changed, which resulted in cell death.
The integrality of cell membrane
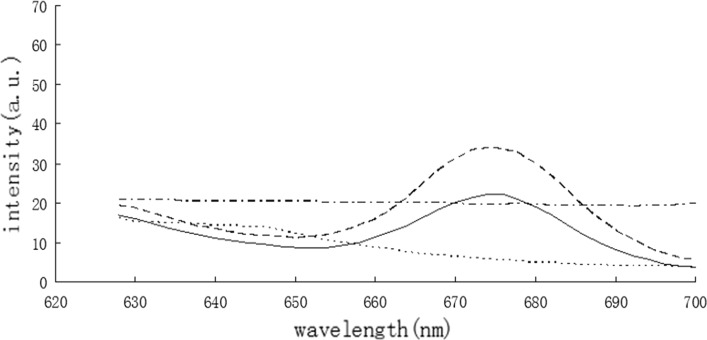
The function of the cell membrane was to segregate intracellular, extracellular components and to prevent the entry of macromolecular substances into the cell. The permeability of the cell membrane could change after treatment with an antibacterial agent. The effect on the cell membrane in response to BPPE was observed by fluorescence spectrometry with a PI tag (Fig. 1). PI cannot normally pass through the cell membrane. The fluorescence intensity of the BPPE treatment groups were significantly higher than that of the control group, which indicated that the treated bacterial cells were damaged and the cell membrane permeability was increased.
Fig. 1.
The fluorescence spectra of PI in cells treated with the BPPE. L. monocytogenes: the antimicrobials (solid line), control (dashed line); S. typhimurium: the antimicrobials (solid line), control (dashed line)
Results of the present study showed that BPPE can increase the permeability of cell membrane, which was consistent with those of previous studies (Liu et al. 2015), indicating that BPPE combined with PL and Nisin could significantly damage the cell membrane of Bacillus subtilis. Ananta et al. (2004) found that the fluorescence dot plot of the bacterial cells treated with high pressure differed with that of the control groups and the heat-inactivated cells. Their results showed that the membranes were ruptured and the cells were labeled by PI throughout the population.
Release of 260 and 280 nm UV-absorbing materials
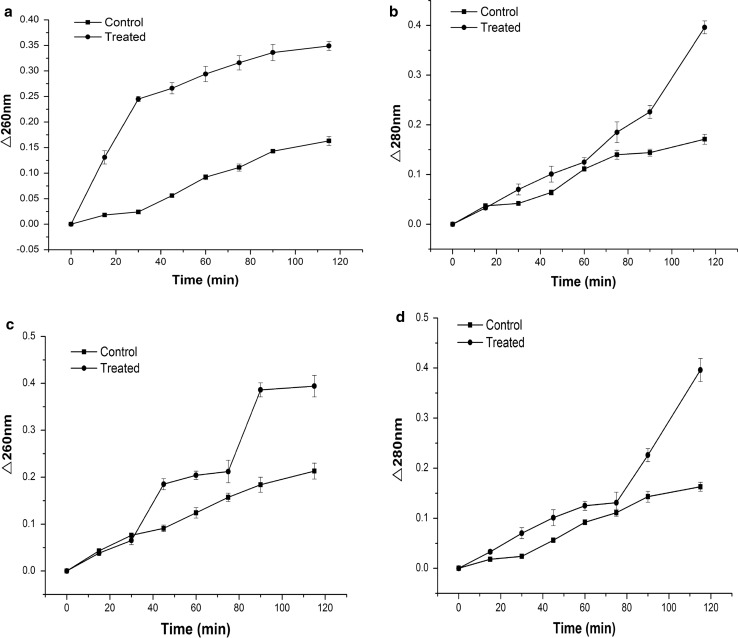
The leaching of 260 and 280 nm UV-absorbing materials (especially nucleic acids and proteins) was monitored for 115 min (Fig. 2). The absorbance at 260 and 280 nm was increased with BPPE treatment. The increasing fast phases of 260 and 280 nm UV-absorbing materials about L. monocytogenes were 0–30 and 75–115 min, respectively. The increasing fast phases of 260 and 280 nm UV-absorbing materials about S. typhimurium were 60–90 and 75–115 min, respectively. Therefore, the nucleic acid and protein were outside the bacterial cell. BPPE altered the permeability of the cell membrane, thereby causing the leaching of nucleic acids and protein. These absorbing materials were obligatory in the bacteria and their leaching could cause cell death.
Fig. 2.
The leakage of UV260 and UV280 absorbing material in L. monocytogenes (a, b) and S. typhimurium (c, d). Data markers represent average (n = 3) ± standard deviation
The BPPE treatment caused leaching of 260 and 280 nm UV-absorbing materials in the present study. These results were consistent with earlier results (Karsha and Lakshmi 2010), indicating that black pepper altered the membrane permeability of S. aureus, thereby resulting in the leakage of nucleic acids and proteins which caused cell death. These results were consistent with these findings of Phongphakdee and Nitisinprasert (2015), who observed that the absorbance of the treated solution was higher than that of the control or the ethanol-treated cells at 260 nm. The release of genetic materials and proteins suggested that BPPE could alter the cell membrane permeability and inhibit the growth of bacteria.
The activity of Na+/K+-ATPase
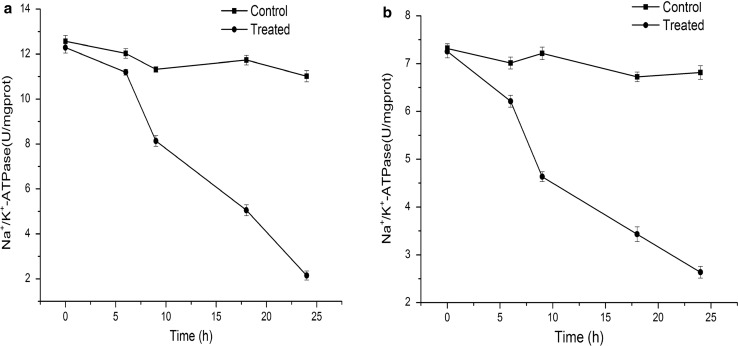
The solubility of antibacterial agent in the membrane phase and the extent of membrane damage can be used to evaluate antimicrobial activity. Natural preservatives inhibit the growth of microorganisms by damaging the cell membrane (Trigui et al. 2013). Several indicators, such as intracellular enzymes, reducing sugar, and phosphates, demonstrate membrane damage. The leaching of potassium and sodium ions evidenced the damage of bacterial cells. Na+/K+-ATPase was an endoenzyme, which promoted the production of ATP at the cell membrane (Kato et al. 2002). The activity of Na+/K+-ATPase on the cell membrane was described in Fig. 3. BPPE showed the strongest inhibition. The ATPase activities were only 2.149 ± 0.201 and 2.636 ± 0.124, respectively, when L. monocytogenes and S. typhimurium were incubated with BPPE at MIC for 24 h.
Fig. 3.
The activity of Na+/K+-ATPase in L. monocytogenes (a) and S. typhimurium (b). Data markers represent average (n = 3) ± standard deviation
Na+/K+-ATPase was a common intracellular enzyme in the membrane of most bacterial cells; this enzyme maintains the balance between the K+ and Na+ concentrations in the cytoplasm. Liu and Pei (2015) found that Nisin could inhibit Na+/K+-ATPase and Ca++/Mg++-ATPase that would cause the lack of ATP for membrane active transport. These results indicated that BPPE destroyed cell membrane and inhibited the ATPase activity, thereby preventing the cell from absorbing the nutrient, limiting the pH regulation and ion transportation (Petrosyan et al. 2015).
Electron microscopic observation
To investigate the morphology of bacteria in response to BPPE, the treated and control bacteria were observed by SEM as shown in Figs. 4 and 5. The cell membrane maintained material and energy balance, which were important for maintaining normal bacterial activity (Li et al. 2010). The BPPE treated bacteria were observed by SEM to confirm the antimicrobial efficacy of BPPE and the morphological changed in the appearance of cells.
Fig. 4.
Effect of black pepper ether extract on the morphology of L. monocytogenes [control (a); ethanol (c 8 h, e 24 h); BPPE (b 8 h, d 24 h)]
Fig. 5.
Effect of black pepper ether extract on the cell morphology of S. typhimurium [control (a); ethanol (c 8 h, e 24 h); BPPE (b 8 h, d 24 h)]
The bacterial cells of the blank control (Figs. 4a, 5a) and the ethanol treatment (for 8 h) groups (Figs. 4c, 5c) of L. monocytogenes and S. typhimurium have normal morphologies. The bacterial cells treated with BPPE (for 8 h) at MIC values were slightly damaged compared with the blank control cells, which have smooth surfaces and relatively intact outer layers (Figs. 4b, 5b). The bacteria cells treated with BPPE (for 24 h) exhibited severely damage compared with the ethanol-treated cells (for 24 h), which had cracked, rough surfaces and mostly fragmented bacteria (Figs. 4d, 5d). By contrast, the effect of ethanol on the cell wall (for 24 h) was slight as compared with that of BPPE (Figs. 4e, 5e).
These findings were consistent with a report on a black pepper chloroform extract, where control E. coli and S. aureus cells appeared smooth and rounded in SEM analysis, whereas those of the treated cells exhibited cell adhesion, unclear boundaries, and fragmented membranes (Zou et al. 2015). The E. coli cells were flattened and lose their cellular integrity after treatment with graphite oxide or reduced graphene oxide (Liu et al. 2011).
Conclusion
This study described the antimicrobial activity of organic compounds in black pepper against L. monocytogenes and S. typhimurium in detail. The MICs of BPPE against L. monocytogenes and S. typhimurium were 0.625 and 1.25 mg/ml, respectively. The cell wall experiment showed that the cell wall integrity was destroyed. The macromolecular substances passed through the cell wall to react on the cell membrane. The antibacterial components of black pepper destroyed the cell membrane by limiting the enzyme activity and changing the permeability, thereby allowing antibacterial agents into the bacteria cell. The activity of Na+/K+-ATPase decreased, so that the antibacterial components of black pepper could restrain the energy metabolism of bacteria. SEM revealed that the bacterial cells fractured and accumulated. Therefore, the mechanism of BPPE inhibited the growth of L. monocytogenes and S. typhimurium by damaging the cell wall and cell membrane, decreasing the enzyme activity and destroying the cell morphology of bacteria. This study provides an approach for developing convenient and efficient antimicrobial agents in the food or pharmaceutical industries, which is of great significance to food security.
Acknowledgements
This research was supported by the National Natural Science Foundation of China (31640061) and Dr Foundation of Hainan University (kyqd1224).
Abbreviation
- BPPE
Black pepper petroleum ether extract
Contributor Information
Hui Tang, Email: hndxtanghui@163.com.
Wenxue Chen, Phone: +86-139-7612-1821, Email: hnchwx@163.com.
Zu-Man Dou, Email: dzman320@163.com.
Ronghao Chen, Email: crh739754055@163.com.
Yueying Hu, Email: hnhuyy@163.com.
Weijun Chen, Email: chenwj@nwu.edu.cn.
Haiming Chen, Email: hmchen168@126.com.
References
- Abd El Mageed MA, Mansour AF, El Massry KF, Ramadan MM, Shaheen MS. The effect of microwaves on essential oils of white and black pepper (Piper nigrum L.) and their antioxidant activities. J Essent Oil Bear Plants. 2011;14:214–223. doi: 10.1080/0972060X.2011.10643924. [DOI] [Google Scholar]
- Abou-Elkhair R, Ahmed H, Selim S. Effects of black pepper (Piper nigrum), turmeric powder (Curcuma longa) and coriander seeds (Coriandrum sativum) and their combinations as feed additives on growth performance, carcass traits, some blood parameters and humoral immune response of broiler chickens. Asian Austr J Anim Sci. 2014;27:847. doi: 10.5713/ajas.2013.13644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ananta E, Heinz V, Knorr D. Assessment of high pressure induced damage on Lactobacillus rhamnosus GG by flow cytometry. Food Microbiol. 2004;21:567–577. doi: 10.1016/j.fm.2003.11.008. [DOI] [Google Scholar]
- Bunthof CJ, van den Braak S, Breeuwer P, Rombouts FM, Abee T. Rapid fluorescence assessment of the viability of stressed Lactococcus lactis. Appl Environ Microb. 1999;65:3681–3689. doi: 10.1128/aem.65.8.3681-3689.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cetin-Karaca H, Newman MC. Antimicrobial efficacy of plant phenolic compounds against Salmonella and Escherichia coli. Food Bioscience. 2015;11:8–16. doi: 10.1016/j.fbio.2015.03.002. [DOI] [Google Scholar]
- Chaudhry N, Tariq P. Bactericidal activity of black pepper, bay leaf, aniseed and coriander against oral isolates. Pak J Pharm Sci. 2006;19:214–218. [PubMed] [Google Scholar]
- Cui Y, Zhao Y, Tian Y, Zhang W, Lü X, Jiang X. The molecular mechanism of action of bactericidal gold nanoparticles on Escherichia coli. Biomaterials. 2012;33:2327–2333. doi: 10.1016/j.biomaterials.2011.11.057. [DOI] [PubMed] [Google Scholar]
- Cui H, Zhang X, Zhou H, Zhao C, Xiao Z, Lin L, Li C. Antibacterial properties of nutmeg oil in pork and its possible mechanism. J Food Saf. 2015;35:370–377. doi: 10.1111/jfs.12184. [DOI] [Google Scholar]
- Dorman H, Deans S. Antimicrobial agents from plants: antibacterial activity of plant volatile oils. J Appl Microbiol. 2000;88:308–316. doi: 10.1046/j.1365-2672.2000.00969.x. [DOI] [PubMed] [Google Scholar]
- Ghori I, Ahmad S. Antibacterial activities of honey, sandal oil and black pepper. Pak J Bot. 2009;41:461–466. [Google Scholar]
- Hu LS, Hao CY, Fan R, Wu BD, Tan LH, Wu HS (2015) De novo assembly and characterization of fruit transcriptome in black pepper (Piper nigrum) PLoS ONE 10 [DOI] [PMC free article] [PubMed]
- Jin YY, Qian DY, Du QZ. Preparation of bioactive amide compounds from black pepper by countercurrent chromatography and preparative HPLC. Ind Crops Prod. 2013;44:258–262. doi: 10.1016/j.indcrop.2012.11.019. [DOI] [Google Scholar]
- Karsha PV, Lakshmi OB. Antibacterial activity of black pepper (Piper nigrum Linn.) with special reference to its mode of action on bacteria. Indian J Nat Prod Resour. 2010;1:213–215. [Google Scholar]
- Kato M, Hayashi R, Tsuda T, Taniguchi K. High pressure-induced changes of biological membrane. Eur J Biochem. 2002;269:110–118. doi: 10.1046/j.0014-2956.2002.02621.x. [DOI] [PubMed] [Google Scholar]
- Kayali HA, Tarhan L, Sazak A, Sahin N. Carbohydrate metabolite pathways and antibiotic production variations of a novel Streptomyces sp. M3004 depending on the concentrations of carbon sources. Appl Biochem Biotechnol. 2011;165:369–381. doi: 10.1007/s12010-011-9256-5. [DOI] [PubMed] [Google Scholar]
- Klotz B, Manas P, Mackey BM. The relationship between membrane damage, release of protein and loss of viability in Escherichia coli exposed to high hydrostatic pressure. Int J Food Microbiol. 2010;137:214–220. doi: 10.1016/j.ijfoodmicro.2009.11.020. [DOI] [PubMed] [Google Scholar]
- Li W-R, Xie X-B, Shi Q-S, Zeng H-Y, You-Sheng O-Y, Chen Y-B. Antibacterial activity and mechanism of silver nanoparticles on Escherichia coli. Appl Microbiol Biot. 2010;85:1115–1122. doi: 10.1007/s00253-009-2159-5. [DOI] [PubMed] [Google Scholar]
- Liu S, et al. Antibacterial activity of graphite, graphite oxide, graphene oxide, and reduced graphene oxide: membrane and oxidative stress. ACS Nano. 2011;5:6971–6980. doi: 10.1021/nn202451x. [DOI] [PubMed] [Google Scholar]
- Liu H, Pei H, Han Z, Feng G, Li D. The antimicrobial effects and synergistic antibacterial mechanism of the combination of ε-Polylysine and nisin against Bacillus subtilis. Food Control. 2015;47:444–450. doi: 10.1016/j.foodcont.2014.07.050. [DOI] [Google Scholar]
- Liu G, Song Z, Yang X, Gao Y, Wang C, Sun B. Antibacterial mechanism of bifidocin A, a novel broad-spectrum bacteriocin produced by Bifidobacterium animalis BB04. Food Control. 2016;62:309–316. doi: 10.1016/j.foodcont.2015.10.033. [DOI] [Google Scholar]
- Nair KP. The agronomy and economy of black pepper (Piper nigrum L.)—the “king of spices”. Adv Agron. 2004;82:271–389. doi: 10.1016/S0065-2113(03)82005-9. [DOI] [Google Scholar]
- Nikolic M, Stojkovic D, Glamoclija J, Ciric A, Markovic T, Smiljkovic M, Sokovic M. Could essential oils of green and black pepper be used as food preservatives? J Food Sci Technol Mysore. 2015;52:6565–6573. doi: 10.1007/s13197-015-1792-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomoto M, Ohsawa M, Wang H-L, Chen C-C, Yeh K-W. Purification and characterization of extracellular alkaline phosphatase from an alkalophilic bacterium. Agric Biol Chem. 2016;52:1643–1647. [Google Scholar]
- Petrosyan M, Shcherbakova Y, Sahakyan N, Vardanyan Z, Poladyan A, Popov Y, Trchounian A. Alkanna orientalis (L.) Boiss. plant isolated cultures and antimicrobial activity of their extracts: phenomenon, dependence on different factors and effects on some membrane-associated properties of bacteria. Plant Cell Tissue Organ Culture. 2015;122:727–738. doi: 10.1007/s11240-015-0806-3. [DOI] [Google Scholar]
- Phongphakdee K, Nitisinprasert S. Combination inhibition activity of nisin and ethanol on the growth inhibition of pathogenic gram negative bacteria and their application as disinfectant solution. J Food Sci. 2015;80:M2241–M2246. doi: 10.1111/1750-3841.13015. [DOI] [PubMed] [Google Scholar]
- Rajmohan K, Soni KB, Swapna A, Nazeem PA, Suku SS. Use of copper sulphate for controlling systemic contamination in black pepper (Piper nigrum L.) cultures. J Food Agric Environ. 2010;8:569–571. [Google Scholar]
- Srinivasan K. Black pepper and its pungent principle-piperine: a review of diverse physiological effects. Crit Rev Food Sci Nutr. 2007;47:735–748. doi: 10.1080/10408390601062054. [DOI] [PubMed] [Google Scholar]
- Tian F, Li B, Ji B, Zhang G, Luo Y. Identification and structure—activity relationship of gallotannins separated from Galla chinensis. LWT Food Sci Technol. 2009;42:1289–1295. doi: 10.1016/j.lwt.2009.03.004. [DOI] [Google Scholar]
- Trigui M, Ben Hsouna A, Tounsi S, Jaoua S. Chemical composition and evaluation of antioxidant and antimicrobial activities of Tunisian Thymelaea hirsuta with special reference to its mode of action. Ind Crops Prod. 2013;41:150–157. doi: 10.1016/j.indcrop.2012.04.011. [DOI] [Google Scholar]
- Valle DL, Jr, Cabrera EC, Puzon JJM, Rivera WL. Antimicrobial activities of methanol, ethanol and supercritical CO2 extracts of Philippine Piper betle L. on clinical isolates of gram positive and gram negative bacteria with transferable multiple drug resistance. PLoS ONE. 2016;11:e0146349. doi: 10.1371/journal.pone.0146349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Aguilar ZP, Qu F, Xu H, Xu HY, Wei H. Enhanced antimicrobial activity of silver nanoparticles-Lonicera Japonica Thunb combo. IET Nanobiotechnol. 2016;10:28–32. doi: 10.1049/iet-nbt.2015.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi SM, Zhu JL, Fu LL, Li JR. Tea polyphenols inhibit Pseudomonas aeruginosa through damage to the cell membrane. Int J Food Microbiol. 2010;144:111–117. doi: 10.1016/j.ijfoodmicro.2010.09.005. [DOI] [PubMed] [Google Scholar]
- Yong AL, Ooh KF, Ong HC, Chai TT, Wong FC. Investigation of antibacterial mechanism and identification of bacterial protein targets mediated by antibacterial medicinal plant extracts. Food Chem. 2015;186:32–36. doi: 10.1016/j.foodchem.2014.11.103. [DOI] [PubMed] [Google Scholar]
- Zappa S, Rolland JL, Flament D, Gueguen Y, Boudrant J, Dietrich J. Characterization of a highly thermostable alkaline phosphatase from the euryarchaeon Pyrococcus abyssi. Appl Environ Microbiol. 2001;67:4504–4511. doi: 10.1128/AEM.67.10.4504-4511.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarai Z, Boujelbene E, Salem NB, Gargouri Y, Sayari A. Antioxidant and antimicrobial activities of various solvent extracts, piperine and piperic acid from Piper nigrum. LWT Food Sci Technol. 2013;50:634–641. doi: 10.1016/j.lwt.2012.07.036. [DOI] [Google Scholar]
- Zarringhalam M, Zarringhalam J, Shadnoush M, Rezazadeh S, Tekieh E. Inhibitory effect of black and red pepper and thyme extracts and essential oils on enterohemorrhagic Escherichia coli and DNase activity of Staphylococcus aureus. Iran J Pharm Res. 2013;12:363–369. [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Liu X, Wang Y, Jiang P, Quek S. Antibacterial activity and mechanism of cinnamon essential oil against Escherichia coli and Staphylococcus aureus. Food Control. 2016;59:282–289. doi: 10.1016/j.foodcont.2015.05.032. [DOI] [Google Scholar]
- Zou L, Hu YY, Chen WX. Antibacterial mechanism and activities of black pepper chloroform extract. J Food Sci Technol Mysore. 2015;52:8196–8203. doi: 10.1007/s13197-015-1914-0. [DOI] [PMC free article] [PubMed] [Google Scholar]