Fig. 1.
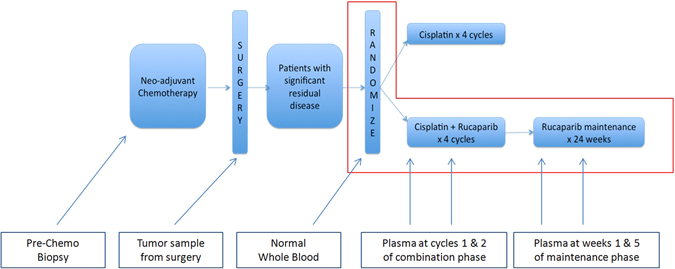
Trial schema for BRE09-146.BRE09-146 was a Phase II clinical trial to evaluate 2-year disease-free survival (DFS) in TNBC patients, treated with either Cisplatin (Arm A) or Cisplatin in combination with PARP inhibitor Rucaparib (Arm B) after neoadjuvant chemotherapy. Tumor tissue, whole blood, and plasma from four time points after surgery were collected as indicated. In this trial, plasma samples were collected only in Arm B of (the area enclosed by the red rectangle). Plasma samples were collected at four timepoints: Cycles 1 and 2 of the combination phase, and during weeks 1 and 5 of the maintenance phase
