Figure 6.
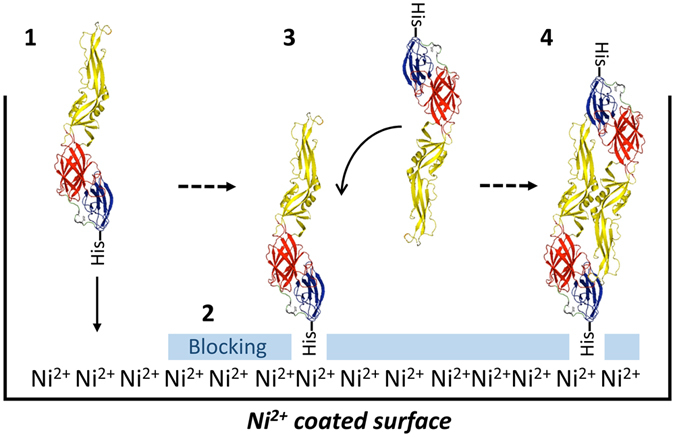
Schematic representation of the postulated model for sRecE-dimer assembly from immobilized monomers. (1) sRecE is chelated to Ni2+-coated plates. The immobilization of sRecE at its C-terminal end presumably locks the protein in a specific conformation. (2) The plates are subsequently blocked and (3) reloaded with sRecE at high protein concentrations. (4) This enables interaction of the immobilized sRecE with the reloaded proteins and generates quaternary epitopes that can be recognized by E-dimer epitope dependent mAbs.
