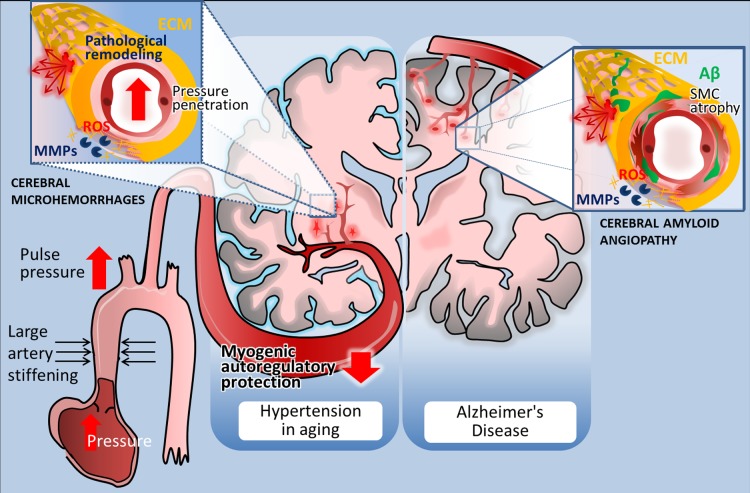
Fig. 2.
Distribution and pathogenesis of cerebral microhemorrhages (CMHs) associated with hypertension and Alzheimer’s disease in elderly patients. Left: in elderly patients CMHs associated with hypertensive vasculopathy typically affect the small perforating end-arteries located in the deep gray nuclei, brain stem, cerebellum, and deep white matter. The scheme highlights potential factors determining location and mechanisms involved in microvascular fragility. Accordingly, age-related large conduit artery stiffening increases pulsatile pressure, which can penetrate into the vulnerable portion of the microcirculation due to an impairment of myogenic autoregulatory protection in the proximal resistance arteries. The predilection of brain regions to hypertension-induced CMHs is determined by the branching pattern of penetrating arterioles. Increased pressure-induced vascular reactive oxygen species (ROS) production activates matrix metalloproteinases (MMPs), which degrade collagens and other components of the extracellular matrix (ECM), compromising the structural integrity of the cerebral microvasculature and promoting CMHs (inset). Right: in Alzheimer’s disease, CMHs develop in vessels affected by cerebral amyloid angiopathy (CAA). Location of the CMHs is determined by the predilection of brain regions for CAA pathology, which preferentially affects the small arteries and arterioles located in the cerebral cortex and at the junction of white and gray matter. The scheme depicts that deposition of Aβ in the vascular wall (green) promotes vascular smooth muscle cell atrophy and oxidative stress and exacerbates MMP-mediated degradation of the ECM, compromising the structural integrity of the vessels. The model predicts that because similar cellular mechanisms are involved in the pathogenesis of CMHs in both conditions, similar interventions should be effective for prevention as well.