Fig. 6.
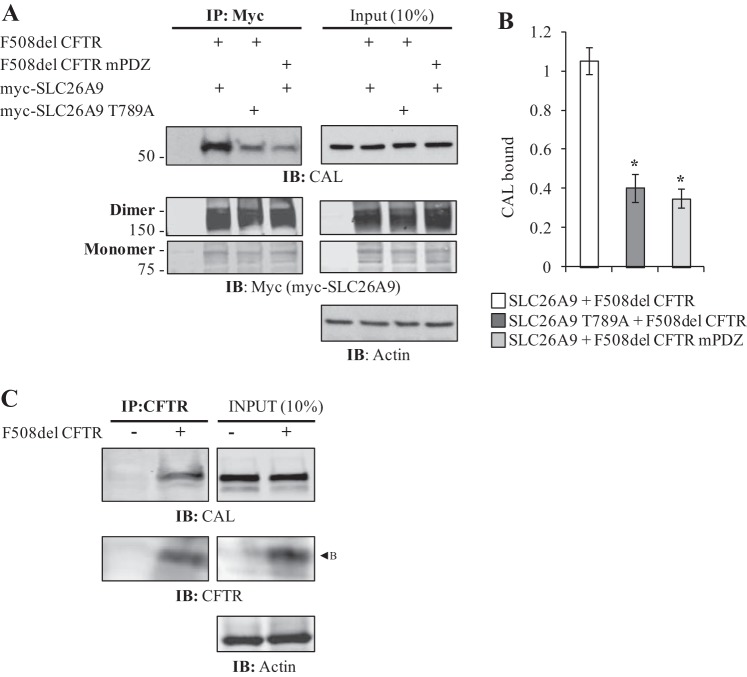
The increase in CAL co-IP shown in Fig. 4B requires that both SLC26A9 and F508del CFTR have intact PDZ motifs. A: mutation of either myc-SLC26A9’s or F508del CFTR’s PDZ motif at the −2 position threonine abrogates the CFTR-induced increase in CAL co-IP. This mutation does not eliminate the PDZ motif from either protein, although the −2 position Thr is central to PDZ domain binding affinity (17). Left panels, IP; right panels, Input. Both monomers and dimers of myc-SLC26A9 are shown. Samples were not treated with forskolin before cell lysis. B: quantification of CAL binding indicates both proteins require intact PDZ motifs to increase myc-SLC26A9’s ability to co-IP CAL. Normalized by total immunoprecipitated myc-SLC26A9 (monomer plus dimer). Averages from 3 independent experiments, *P < 0.05. C: immature (band B) F508del CFTR co-IPs CAL when expressed alone. Left panels, IP; right panels, Input.