Fig. 8.
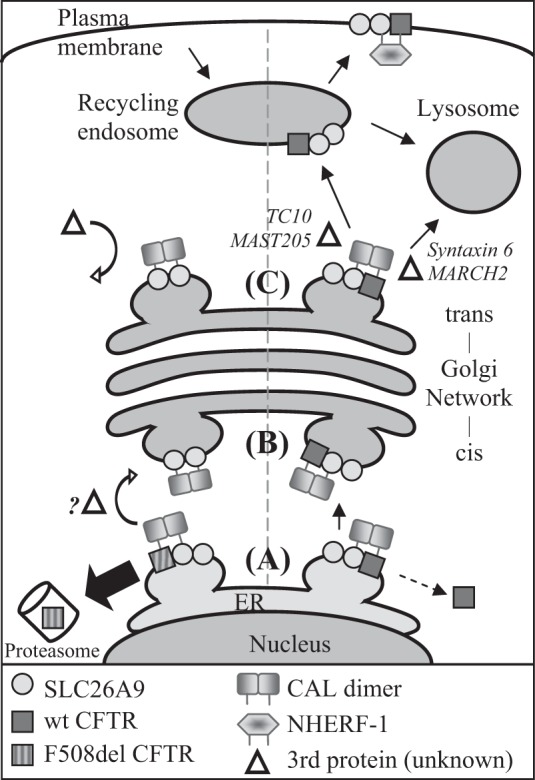
A model demonstrating potential PDZ domain protein interactions for coexpressed SLC26A9 and CFTR. Left side, F508del CFTR coexpression; right side, wt CFTR coexpression. A: the proteins commence their interaction in the ER, where the CAL dimer binds CFTR and SLC26A9. Misfolded F508del CFTR is removed from the complex and undergoes ERAD, leaving half of the CAL dimer unbound. If SLC26A9 is dimerized at this stage, removal of F508del CFTR may result in a CAL dimer-SLC26A9 dimer complex. A portion of wt CFTR may also be degraded at this stage (see text). Step B: the CAL complex traffics to and through the Golgi complex, either as a CAL-SLC26A9 dimer (F508del CFTR coexpression), or a CAL-SLC26A9-wt CFTR complex. Step C: at the TGN, the CAL-SLC26A9-wt CFTR complex may be disrupted by additional proteins which interact with CFTR, such as TC10 (14) or MAST205 (47). Since an individual SLC26A9 PDZ motif has relatively weak affinity for CAL (Table 1), the complex is released from the TGN and continues trafficking to the recycling endosome and ultimately, plasma membrane. Conversely, the lack of F508del CFTR in the CAL-SLC26A9 dimer complex impedes the recruitment of additional proteins, which, combined with a greater affinity between CAL and SLC26A9 due to avidity, halts the forward trafficking of SLC26A9.
