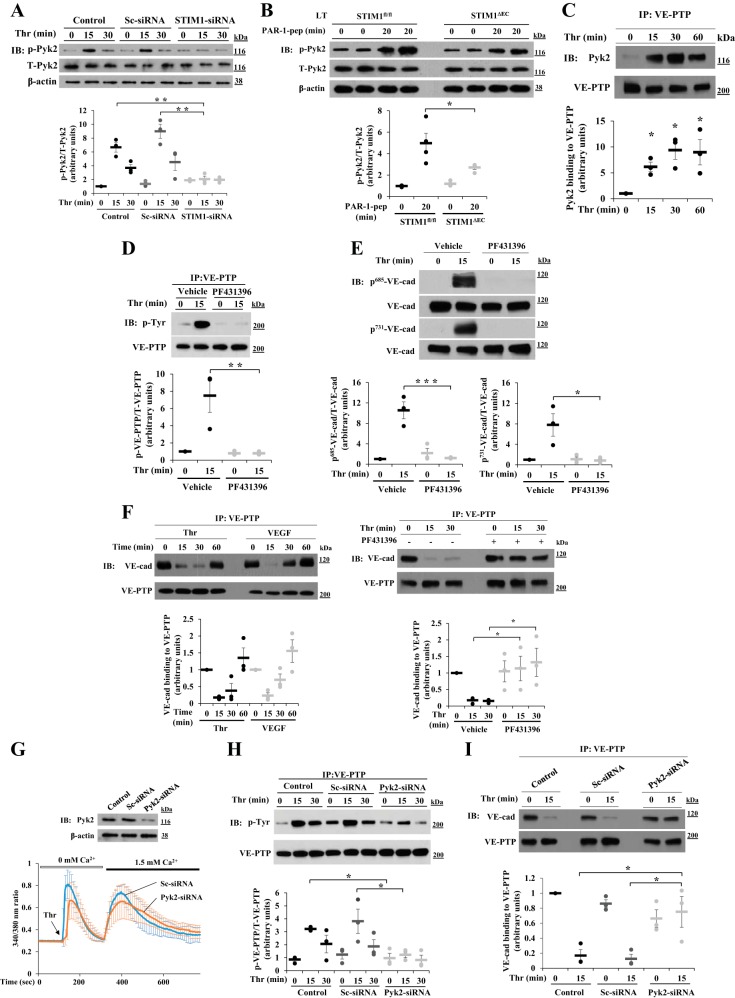
Fig. 3.
STIM1-dependent Pyk2 activation induces tyrosine phosphorylation of VE-PTP in ECs. A: HLMVECs transfected with sc-siRNA or STIM1-siRNA as in Fig. 1 were used to measure thrombin-induced phosphorylation of Pyk2 at Y402 as a measure of Pyk2 activation. Quantified results are means ± SE of 3 experiments and are presented as the ratio of phosphorylated:total protein. **P < 0.01; compared with control or sc-siRNA-treated cells. B: Stim1fl/fl and Stim1ΔEC mice received PAR-1 agonist peptide as described in Fig. 2B. 0 and 20 min after administration of PAR-1 agonist peptide, the lungs were used for IB to determine tyrosine phosphorylation of Pyk2 at Y402. Quantified results are means ± SE and are shown as the ratio of phosphorylated:total protein. n = 4 mice for each group. *P < 0.05; Stim1fl/fl vs. Stim1ΔEC. C: HLMVECs exposed to thrombin were immunoprecipitated with VE-PTP pAb and blotted with anti-Pyk2 pAb. Quantified results are means ± SE of 3 experiments. *P < 0.05; control vs. thrombin-treated. D: HLMVECs were pretreated with PF431396 (1 μM) or vehicle (DMSO) for 15 min and then challenged with thrombin (25 nM). After thrombin treatment, cell lysates were immunoprecipitated with anti-VE-PTP pAb and immunoblotted with a phospho-tyrosine-specific mAb. The blot was reprobed with anti-VE-PTP pAb. E: HLMVECs challenged with thrombin as in D were immunoblotted with the indicated phospho-tyrosine-specific VE-cadherin antibodies. Quantified results are means ± SE of 3 experiments presented as the ratio of phosphorylated:total protein in D and E. *P < 0.05; **P < 0.01; ***P < 0.001; control (vehicle) vs. PF431396. F: HLMVECs challenged with thrombin (25 nM) or VEGF A (50 ng/ml) were immunoprecipitated with anti-VE-PTP pAb and immunoblotted with anti-VE-cadherin pAb (left). Quantified results are means ± SE of 3 experiments. HLMVECs were pretreated with PF431396 (1 μM) or vehicle (DMSO) for 30 min and then exposed to thrombin; cells were immunoprecipitated with anti-VE-PTP pAb and immunoblotted with anti-VE-cad pAb (right). Quantified results from 3 experiments are shown. *P < 0.05; control vs. PF431396-treated. G: LECs from wild-type (C57BL/6) mice transfected with 100 nM sc-siRNA or Pyk2-siRNA were immunoblotted with anti-Pyk2 pAb to determine Pyk2 expression (top), or cells were used to measure thrombin-induced ER-store Ca2+ release and Ca2+ release-activated Ca2+ entry (bottom). Values are means ± SD, n = 15 cells per group. H: control LECs, sc-siRNA-transfected LECs, and Pyk2-siRNA-transfected LECs were exposed to thrombin for 0, 15, and 30 min. After thrombin treatment, cell lysates were immunoprecipitated with anti-VE-PTP pAb and immunoblotted with anti-phospho-tyrosine mAb. The blot was reprobed with anti-VE-PTP pAb. Quantified data from 3 experiments are presented as the ratio of phosphorylated:total protein. *P < 0.05; significantly different from control or sc-siRNA-transfected. I: control LECs, sc-siRNA-transfected LECs, and Pyk2-siRNA-transfected LECs were exposed to thrombin for 0 and 15 min. After thrombin treatment, cell lysates were immunoprecipitated with anti-VE-PTP pAb and immunoblotted with anti-VE-cad pAb. The blot was reprobed with anti-VE-PTP pAb. Quantified results from 3 experiments are shown. *P < 0.05; significantly different from control or sc-siRNA-transfected.