Figure 2.
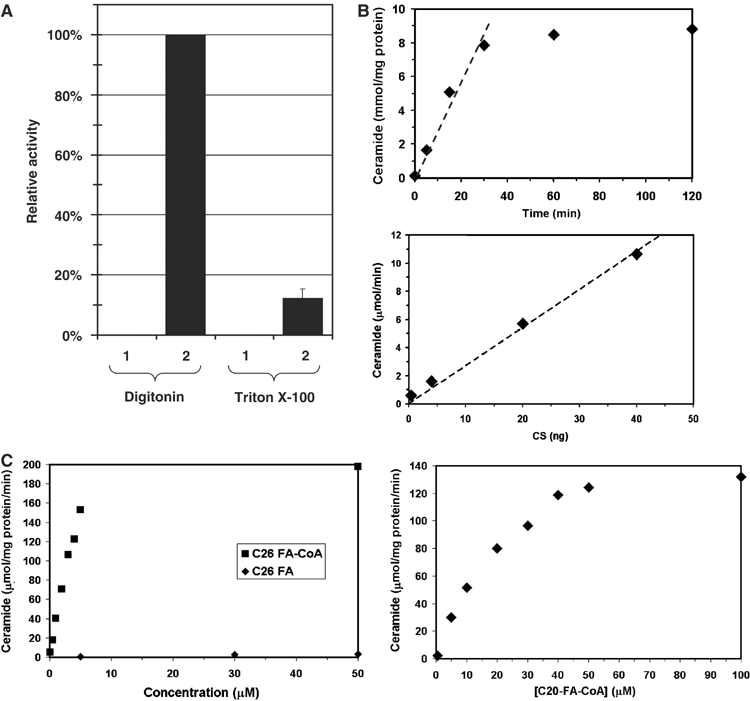
Enzymatic characterization of purified ceramide synthase. Microsomal membranes were solubilized with either digitonin or Triton X-100, and Flag-Lac1p-containing protein complexes were purified by immunoisolation and eluted from the beads. Ceramide synthase activity was measured. (A) Lanes 1, 2: membranes were solubilized with 1% digitonin, lanes 3, 4: membranes were solubilized with 1% Triton X-100. Lane (1) corresponds to experiments with strain RH 5665 and lane (2) to strain RH 5666. (B) Time course (upper panel) and protein concentration dependence (lower panel) of ceramide synthase activity. (C) Fatty acyl-CoA concentration and CoA dependence of ceramide synthase activity. C26 fatty acid substrates are shown in the left panel and C20 fatty acyl-CoA in the right panel.
