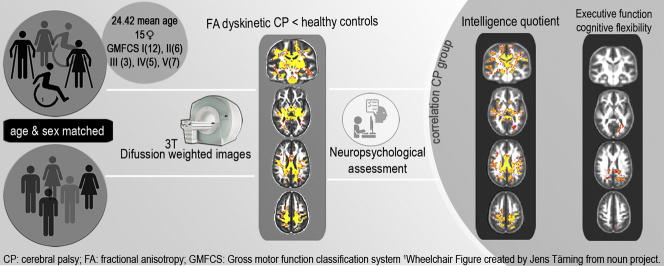
Abstract
Background
Dyskinetic cerebral palsy (CP) is one of the most disabling motor types of CP and has been classically associated with injury to the basal ganglia and thalamus. Although cognitive dysfunction is common in CP, there is a paucity of published quantitative analyses investigating the relationship between white matter (WM) microstructure and cognition in this CP type.
Aims
This study aims (1) to compare brain WM microstructure between people with dyskinetic CP and healthy controls, (2) to identify brain regions where WM microstructure is related to intelligence and (3) to identify brain regions where WM microstructure is related to executive function in people with dyskinetic CP and (4) to identify brain regions where the correlations are different between controls and people with CP in IQ and executive functions.
Patients and methods
Thirty-three participants with dyskinetic CP (mean ± SD age: 24.42 ± 12.61, 15 female) were age and sex matched with 33 controls. Participants underwent a comprehensive neuropsychological battery to assess intelligence quotient (IQ) and four executive function domains (attentional control, cognitive flexibility, goal setting and information processing). Diffusion weighted MRI scans were acquired at 3T. Voxel-based whole brain groupwise analyses were used to compare fractional anisotropy (FA) and of the CP group to the matched controls using a general lineal model. Further general linear models were used to identify regions where white matter FA correlated with IQ and each of the executive function domains.
Results
White matter FA was significantly reduced in the CP group in all cerebral lobes, predominantly in regions connected with the parietal and to a lesser extent the temporal lobes. There was no significant correlation between IQ or any of the four executive function domains and WM microstructure in the control group. In participants with CP, lower IQ was associated with lower FA in all cerebral lobes, predominantly in locations that also showed reduced FA compared to controls. Attentional control, goal setting and information processing did not correlate with WM microstructure in the CP group. Cognitive flexibility was associated with FA in regions known to contain connections with the frontal lobe (such as the superior longitudinal fasciculus and cingulum) as well as regions not known to contain tracts directly connected with the frontal lobe (such as the posterior corona radiata, posterior thalamic radiation, retrolenticular part of internal capsule, tapetum, body and splenium of corpus callosum).
Conclusion
The widespread loss in the integrity of WM tissue is mainly located in the parietal lobe and related to IQ in dyskinetic CP. Unexpectedly, executive functions are only related with WM microstructure in regions containing fronto-cortical and posterior cortico-subcortical pathways, and not being specifically related to the state of fronto-striatal pathways which might be due to brain reorganization. Further studies of this nature may improve our understanding of the neurobiological bases of cognitive impairments after early brain insult.
Graphical abstract
Highlights
-
•
There is widespread loss in the integrity of WM in dyskinetic CP.
-
•
WM integrity is mainly reduced in regions near the parietal cortex.
-
•
IQ is related to FA in the majority of the regions showing a reduced WM integrity.
-
•
Executive function is related with cortico-cortical and posterior cortico-subcortical WM FA.
-
•
Executive function is not associated with WM FA of the fronto-striatal circuit in dyskinetic CP.
1. Introduction
Cerebral palsy (CP) describes a group of permanent disorders of the development of movement and posture, causing activity limitations, that are attributed to non-progressive disturbances that occurred in the developing foetal or infant brain (Rosenbaum et al., 2007). The predominant motor types in CP can be classified in three groups: spastic, dyskinetic, and ataxic (Surveillance of Cerebral Palsy in Europe, 2000). Dyskinetic CP is the second largest CP group, comprising between 3 and 15% of CP cases with higher rates among children born at term and among children with normal birth weight (Himmelmann et al., 2009). Together with spastic quadriplegia, people with dyskinetic CP have poorer motor and cognitive outcomes than people with other CP motor types (Sigurdardottir et al., 2008). Most studies of cognition in CP, however, have focused on unilateral CP, and their results may not be generalized to bilateral cases. There are few studies examining cognition and neuroimaging in people with dyskinetic CP (Scheck et al., 2012), possibly due to the challenges associated with performing these assessments in this group of patients.
The main CP subtypes (spastic and dyskinetic) are associated with different brain lesions (Krageloh-Mann and Cans, 2009). Qualitative neuroimaging studies indicate that spastic CP mainly presents periventricular leukomalacia, while dyskinetic CP is associated with damage to the cortical grey matter as well as the basal ganglia and thalamus (Bax et al., 2006). Quantitative neuroimaging studies further indicate that abnormalities also tend to be more severe and diffuse in participants with dyskinetic CP than in participants with spastic CP (Yoshida et al., 2013, Yoshida et al., 2011). Studies of dyskinetic CP have reported cortico-subcortical lesions (Himmelmann and Uvebrant, 2011), white matter (WM) damage (Yoshida et al., 2013, Yoshida et al., 2011), as well as normal or non-specific neuroimaging findings (Towsley et al., 2011).
In addition to motor disorders, one in two people with CP have intellectual disability (Novak et al., 2012) and specific cognitive difficulties have been described as well (Straub and Obrzut, 2009). Studies that have focused on executive functions have reported difficulties with inhibitory control, shifting and categorization (Bottcher, 2010, Christ et al., 2003, Jenks et al., 2009, Kolk and Talvik, 2000) which can explain difficulties in everyday life (Whittingham et al., 2014). Lesions of the basal ganglia and thalamic systems may impair focused attention and executive function (Bottcher, 2010). These brain lesions are frequently described in people with dyskinetic CP (Bax et al., 2006, Himmelmann and Uvebrant, 2011) but only one study has focused on executive function (executive function) and brain magnetic resonance imaging (MRI) in this CP subtype (Laporta-Hoyos et al., 2017). Although executive functions depend on the integrity of the entire brain, they are mainly mediated by the frontal lobes and its connections with posterior and subcortical brain regions (Anderson, 2008, Bodimeade et al., 2013). Analysing the association between executive function and WM microstructure with advanced imaging techniques may help in understanding neuroplastic mechanisms that take place after disturbances in the foetal or infant brain. To date there is a lack of brain imaging studies investigating executive function in dyskinetic CP.
Research in the field of intelligence has emphasized the crucial role of WM tracts underlying fronto-parietal association cortices, such as the superior longitudinal fasciculus (Jung and Haier, 2007). A previous study of our group showed that in dyskinetic CP, intelligence quotient (IQ) was related to the volumes of the basal ganglia, thalamus, and superior longitudinal fasciculus, retrolenticular part of the internal capsule, cingulum and superior corona radiata (Ballester-Plané et al., 2016). That previous study did not aim to analyse microstructure and did not include a control group (Ballester-Plané et al., 2016). Lesions of the thalamus and basal ganglia as well as features of lesions such as lesion type (periventricular leukomalacia vs arterial ischemic stroke) and lesion severity have been associated with the severity of cognitive impairment in spastic CP (Riva et al., 2012).
A recent review concluded that dyskinetic CP should be considered separately to other motor types, looking at the relationship to clinical measures in order to provide further insight into the pathogenesis of this condition (Scheck et al., 2012). Our study aims to investigate how intelligence and executive function relate to brain microstructure in people with dyskinetic CP. To analyse WM integrity and organization, diffusion MRI was used. By probing the random motion of water molecules, measures of fractional anisotropy (FA; often reported as a surrogate marker for WM ‘integrity’) and mean diffusivity (MD) were computed (Basser and Pierpaoli, 1996). There is evidence that decreased FA and increased MD correlate with measures of clinical severity of CP and provide information about corticomotor reorganization (Reid et al., 2016); however networks associated with cognition have been less thoroughly studied (Scheck et al., 2012). Diffusion imaging studies in CP provide new insight into the specific injury and reorganization of WM motor pathways; however, current data are limited and focused on unilateral and spastic diplegia (Rai et al., 2013, Scheck et al., 2015). Furthermore, dyskinetic CP is generally undersampled and is thought to be accompanied by significantly different diffusion properties than unilateral and spastic CP in many brain regions (Scheck et al., 2012).
This study aims (1) to compare brain WM microstructure between people with dyskinetic CP and healthy controls, (2) to identify brain regions where WM microstructure is related to intelligence and (3) to identify brain regions where WM microstructure is related to executive function in people with dyskinetic CP and (4) to identify brain regions where the correlations are different between controls and people with CP in IQ and executive functions.
In this study our primary hypothesis was that FA would be reduced in participants with CP compared to healthy controls in several regions, reflecting impaired WM organization. Our second hypothesis was that, for some of the regions showing significant differences to controls, FA would be correlated with cognitive performance. Specifically, that IQ would correlate with FA in structures such as the superior longitudinal fasciculus and the cingulum, in accordance with previous studies (Ballester-Plané et al., 2016). We further hypothesised that executive function would correlate with FA and MD of the fronto-striatal loop. We expected FA to correlate with executive function performance in different regions in participants with dyskinetic CP and controls, due to compensatory neural development.
2. Materials and methods
2.1. Participants
All procedures performed in the study were in accordance with the ethical standards of the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Ethical approval was obtained by the University of Barcelona's (CBUB) Institutional Ethics Committee, Institutional Review Board (IRB 00003099, assurance number: FWA00004225; http://www.ub.edu/recerca/comissiobioetica.htm) and the Hospital Universitari Vall d'Hebron. Written informed consent was obtained from all participants included in the study or their parents/legal guardian.
The study sample included a subset of participants of a larger project that recruited people with dyskinetic CP from the Hospital Vall d'Hebron (Pediatric Neurology Department and Rehabilitation and Physical Medicine Department) (n = 27), the Hospital Sant Joan de Déu (n = 3) (Neurology Department), the Cerebral Palsy Association ASPACE (n = 1) (Health services and rehabilitation) in Barcelona, Spain as well as from a previous study (n = 2) (Pueyo et al., 2009). Physicians from the above mentioned institutions informed their patients with dyskinetic CP or their parents/legal guardian about the possibility to participate in this research project. Patients where further contacted by phone to check inclusion/exclusion criteria, to explain the participation procedure and to offer to participate in the study. Participants were recruited and data were collected between 2012 and 2015.
Inclusion criteria for the study were (1) clinical diagnosis of CP with predominant dyskinetic features; (2) being older than 6 years; (3) an intelligible yes/no response system; and (4) for the neuropsychological assessment, being able to understand instructions, as evaluated by the Spanish grammar screening test (receptive part) (Toronto, 1973). Exclusion criteria were the presence of severe visual or auditory disability that precludes neuropsychological assessment.
Thirty-three participants with dyskinetic CP aged 6–59 years, 15 female, who satisfied the inclusion criteria were able to successfully undergo an MRI (Table 1). Thirty-three typically developing people without brain pathology and matched by age and sex were also included in all analyses as controls. Inclusion criteria were therefore to have same sex and similar age (± 1 year) as a participant with dyskinetic CP. Controls were ineligible if they were born preterm, were suffering from a neurological or psychiatric disorder, or were illicit substance consumers. The control group was composed of 20 friends and relatives of the participants with CP, while the remaining 13 controls were recruited through advertisements.
Table 1.
Demographics and clinical data of subjects with dyskinetic cerebral palsy and healthy age and sex matched controls.
| CP group | Control group | |
|---|---|---|
| Sex n (female/male) |
15/18 | 15/18 |
| Age Mean (SD)/range |
24.42 (12.61)/6–59 | 24.42 (12.44)/7–59 |
| Gestational age n (< 32 weeks/32–36 weeks/≥37 weeks) |
2/4/27 | 0/0/33 |
| Epilepsy status n (no epilepsy/active/resolved) |
23/8/2 | 33/0/0 |
| Aetiology, n |
– |
|
| HIE | 14 | |
| Intra-cranial haemorrhage/infarction/hydrocephalus | 3 | |
| Infection | 1 | |
| Kernicterus | 1 | |
| Unclassifiable | 14 | |
| Gross motor function (GMFCS) (n) | I (12) II (6) III (3) IV (5) V (7) |
– |
| Fine motor function (MACS) (n) | I (5) II (8) III (11) IV (2) V (7) |
– |
| Communication (CFCS) (n) | I (14) II (13) III (2) IV (4) V (0) |
– |
| Motor distribution (tetraplegia/hemiplegia/monoplegia) | 28/4/1 | – |
CFCS: Communication function classification system; CP: cerebral palsy; GMFCS: Gross motor function classification system; HIE: hypoxic-ischemic encephalopathy; MACS: Manual ability classification system; SD: standard deviation.
2.2. Neuropsychological assessment
Tests used were carefully chosen to allow the majority of the participants to answer in an autonomous way. All but two of the tests were computerized and allowed for use of assistive technology for communication. Participants were encouraged to use the response technique best suited to their degree of disability and the communication devices they normally used.
2.2.1. Intelligence quotient
The Raven's coloured progressive matrices (RCPM) test was used to measure intelligence quotient (Raven et al., 2001). This test is recommended for people with physical disabilities, aphasia, deafness or CP (Strauss et al., 2006), and has been shown to be sensitive to brain structure in dyskinetic CP (Ballester-Plané et al., 2016). The RCPM have also been commonly used in research with healthy controls (Strauss et al., 2006). Raw scores were used in the neuroimaging analyses. Raw scores range from 0 to 36 and can be converted into IQ scores using normative data for children (Raven et al., 2001) and normative data for adults (Measso et al., 1993). For adolescents, we used a linear interpolation, as previously described (Ballester-Plané et al., 2016). The different ways of response used were: orally (saying the item number), pointing (with the finger, hand or an adapted pointer on the head), or, in cases in where an autonomous response was not possible, the examiner indicated each response alternative while asking the participant if it was his/her choice. Subjects further answered “yes” or “no” by means of vocalisations, movement of head, facial miming or gestures with other parts of the body.
2.2.2. Executive function
A comprehensive neuropsychological test battery was used to assess the four domains of executive function according to Anderson (2008): attentional control, cognitive flexibility, goal setting and information processing.
Attentional control was assessed using the Stop signal task (SST) of the Cambridge neuropsychological test automated battery (CANTAB) (Cambridge Cognition, 1999). The test instructs participants to respond as fast as possible to a simple arrow stimulus on a computer screen. The participant was told to press the left button when they see a left-pointing arrow and the right button when they see a right-pointing arrow. The task was switch adapted and participants were therefore able to respond pressing the buttons by hand, cheek, chin, head or feet to allow autonomous responses. On some trials, an auditory stop signal was presented, and participants are instructed to try and stop or inhibit their response. In the original version of the test, at the end of every assessed block, a feedback screen was displayed showing a graphical representation of the participant's performance. These resting stops were removed in the present study in order to increase the attentional component of the task. The number of correct responses on “stop” and “go” trials represents the total score. Higher scores indicate better performance.
Cognitive flexibility was assessed using the 64-item computerized version of the Wisconsin card sorting test (WCST) (Kongs et al., 2000). This task, which is one of the most widely used tests of executive function in clinical and experimental neuropsychology, consists of four reference cards and 64 response cards with geometric figures that vary in colour, shape and number. The participant has to pair each response card with one of the four reference cards and discover the correct classification principle by trial and error and the computer feedback (Kongs et al., 2000). The score used was the number of perseverative errors. Higher scores indicate poorer performance and raw scores were converted into z scores using normative data provided with the test manual. To access the test, a mouse/joystick (controlled by hand or with the chin) and one switch (pressed by hand, cheek, head or foot) were used. In cases where an autonomous response was not possible, the examiner indicated the various response alternatives while asking the participant if it was his/her choice, and then the examiner executed the action.
Goal setting was evaluated by means of the Stockings of Cambridge (SOC) test of CANTAB (Cambridge Cognition, 1999). The SOC is a spatial planning test in which the participant is shown two displays containing three coloured balls. The participant must use the balls in the lower display to copy the pattern shown in the upper display. The outcome measure used for the analysis was the number of problems solved in minimum moves (Cambridge Cognition, 1999). Higher scores indicate better performance. Raw scores were converted into z scores using normative data provided with the test manual. To access the test, a mouse/joystick (controlled by hand or with the chin) and one switch (pressed by hand, cheek, head or foot) were used. Some participants responded the test by pointing to the computer screen (with the finger, hand or an adapted pointer on the head). In cases where an autonomous response was not possible, the examiner indicated the various response alternatives while asking the participant if it was his/her choice, and then the examiner executed the action.
Information processing was measured using the performance in a lexical verbal fluency task with three initial-letters (Gramunt-Fombuena and Pen, 2009), a frequently used task in clinical practice. The test requires participants to generate as many words as possible beginning with P, M, and R and the score used was the number of words that the participant were able to say. Higher scores indicate better performance.
2.2.3. Statistical analysis
Descriptive statistics of neuropsychological performance were calculated. The cognitive performances of the CP and control groups against their matched controls were compared using t-tests or Mann–Whitney U tests, depending on the distribution of the data. Statistical analyses of neuropsychological data were performed using SPSS version 24 (IBM SPSS Statistics, IBM Corp. NY, USA). The level of significance was set at p-value < 0.05. Missing data were handled with pairwise deletion.
2.3. Neuroimaging
2.3.1. Image acquisition
MRI data were acquired on a Siemens Magnetom TRIO 3 Tesla scanner (Erlangen, Germany) at the Hospital Universitari Vall d'Hebron (Barcelona, Spain), TQ-engine Tim (45 mT/m, slew rate 200 T/m/s), using a 12 element Tim head array.
Diffusion weighted images were acquired in 30 noncollinear directions and 65 axial slices. Parameters were: 2 mm slice thickness; field of view 240 × 240 mm; TR/TE 8400/90 ms; and acquisition matrix 122 × 122, voxel size 1.96 × 1.96 mm. Images were acquired at b = 1000 s/mm2, along with one minimally diffusion weighted image (b = 0). The acquisition time was 4:47 min.
In order to minimize movement during MRI, diazepam was administered in 13 patients (dose between 2.5 and 10 mg depending on each participant) and 6 were sedated with pentobarbital and propofol. The drug was prescribed by a physician in accordance with the protocol detailed and reviewed by the ethics committee.
2.3.2. Diffusion preprocessing
An extensive preprocessing procedure was used. Volumes containing within-volume motion or scanner artefacts were visually identified, and excluded from further analysis. Participants whose datasets contained more than three volumes showing motion artefacts were excluded entirely from further analyses. Brain masks where created using FSL BET (Smith, 2002) and further manually edited as required. FSL EDDY (Andersson and Sotiropoulos, 2015) was used to correct for eddy current-induced distortions and head movements between volumes, including rotation of the b-vectors. DTI maps including FA and MD were calculated for each participant using FSL DTIFIT.
2.3.3. Voxelwise analysis
Whole brain groupwise analyses of FA images were carried out using a variation (Schwarz et al., 2013) of the original tract based spatial statistics (TBSS) pipeline (Smith et al., 2006) shown to improve reliability. A study specific template was created from the FA maps of all participants, and FA maps of all participants were subsequently non-linear registered to this template using ANTS-SyN (http://picsl.upenn.edu/software/ants/) (Avants et al., 2012, Fonslow et al., 2013). Greedy SyN was used as the transformation model. Probability mapping was used as the metric, GradStep = 0.25, regularization sigma = 2.0, 4 iterations of template construction, number of iterations per level 30 × 90 × 20.
Registration accuracy was assessed visually, and data with registration errors were excluded from further analysis. Creation of the study specific template was repeated excluding these participants. A Gaussian smoothing kernel with sigma of 2 mm was applied. A WM mask was created by first eroding the average brain mask by 3 voxels, applying a group average FA threshold of 0.2 and retaining only voxels that were non-zero in all participants.
A permutation-based modelling approach (Nichols and Holmes, 2001) implemented in FSL RANDOMISE with 5000 iterations was used to perform all statistical analyses. The resultant statistical maps were thresholded at p < 0.05 corrected for multiple comparisons with threshold-free cluster enhancement (Smith and Nichols, 2009). Although our primary hypotheses were one-tailed, the opposite contrast was also investigated to confirm absence of significant differences. A voxelwise analysis for participants with dyskinetic CP compared to healthy controls was performed to explore differences in FA between the CP and control participants. A correlation between IQ and FA was then performed, controlling for age and sex, for all participants with CP and controls separately to ascertain regions where injury severity was specifically associated with IQ. Finally, four separate general linear models were employed to identify regions where FA correlated with scores in each one of the executive function domains in the CP and control group (controlling for age and sex). All analyses were also further performed for MD (see Supplementary material). Anatomical naming of WM regions containing significant clusters was performed using the John Hopkins University (JHU) WM atlas (Hua et al., 2008) included in FSL. The Harvard-Oxford cortical structural atlas (http://www.cma.mgh.harvard.edu/fsl_atlas.html) was further used to report cortical structures that were close to significant WM results. Only clusters containing at least five voxels are presented.
3. Results
3.1. Demographic and clinical information
From a total of 101 potentially eligible patients, 9 could not be located, 26 refused to participate in the study, 2 were excluded for being treated for substance use disorder, 7 did not meet the criterion of understanding simple instructions, and 5 withdrew from follow-up participation.
Among the 52 remaining cases some were not included in the present study because 2 could not be scanned due to metal devices implanted, 3 refused to undergo MRI, and 5 did not complete scans due to anxiety. From the remaining 42 cases, 6 were excluded due to excessive head movement artefacts or signal-to-noise problems on the MRIs and 3 could not be adequately processed by the software.
The final sample comprised 33 participants with dyskinetic CP aged 6–59 years without visual/auditory abnormalities, able to understand instructions and, at least, able to answer yes/no, as well as 33 age and sex matched healthy controls. The majority of the participants (n = 27; 81%) were born at term as expected in this CP type (Himmelmann et al., 2007). In the CP group 8 (24%) participants had active epilepsy, the most frequent classifiable aetiology being hypoxic-ischemic encephalopathy (n = 14; 42%) and the vast majority had tetraplegia (n = 28; 85%). Participants' gross and fine motor function (GMFCS: Gross motor function classification system, and MACS: manual ability classification system) ranged from I to V and communication (CFCS: Communication function classification system) from I to IV (Table 1).
The neuropsychological performance of the sample is reported in Table 2. Eight participants with dyskinetic CP had an IQ equal to or below 70 (24%) indicating intellectual disability according the criterion A of DSM-5 (American Psychiatric Association, 2013). There was more variability in the CP group in almost all cognitive function scores. The only domain where the control group showed higher variability in their performance was information processing which was measured by a task that requires being able to speak.
Table 2.
Descriptive statistics of neuropsychological performance of subjects with dyskinetic cerebral palsy and age and sex matched healthy controls.
| n | Score | CP group |
Control group |
Differences | ||||
|---|---|---|---|---|---|---|---|---|
| Range | Mean (SD) or Median (IQR) | Range | Mean (SD) or Median (IQR) | |||||
| IQ | Intelligence quotient† | 33 | Raw | 12–36 | 29 (12) | 29–36 | 35 (2) | ⁎ < 0.001; U = 217 |
| IQ | 33–127 | 94 (41) | 95–128 | 113 (7) | ⁎ < 0.001; U = 257 | |||
| EF | Attentional control† | 31a | Raw | 244–316 | 283.60 (12) | 266–312 | 283.84 (14) | NS. U = 462.50 |
| Cognitive flexibility†,+ | 31a | Raw | 4–35 | 8 (7) | 2–23 | 7 (5) | NS. U = − 401 | |
| z | − 2.70–3 | − 0.30 (1.30) | − 2.10–3 | − 0.30 (1.10) | NS. U = 451.50 | |||
| Goal setting | 30a | Raw | 3–12 | 7.93 (2.35) | 6–12 | 9.40 (1.57) | ⁎0.006 t = 2.85 | |
| z | − 2.52–1.91 | 0.02 (1.11) | − 1.04–1.91 | 0.70 (0.76) | 0.007 t = − 2.79 | |||
| Information processing | 26b | Raw | 4–51 | 28.42 (12.60) | 11–68 | 40.04 (13.65) | ⁎0.002 t = 3.19 | |
CP: cerebral palsy; EF: executive function; IQ: intelligence quotient; IQR: interquartile range; NS: no significant; SD: standard deviation. Reasons for missing data: aAnarthria accompanied by very severe motor impairments that preclude to use an appropriate response system for the test used; bAnarthria or severe dysarthria.
⁎Bonferroni correction for multiple-comparison was applied and significance is at level set of 0.006.
†Data in one or both groups is not normally distributed, thus, non-parametric test is applied and median (IQR) is indicated in italic.
+Higher scores indicate worse performance.
3.2. Neuroimaging
3.2.1. Differences between dyskinetic CP and control participants
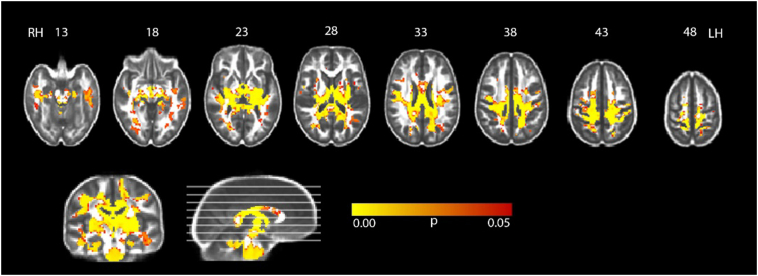
The voxel-based analysis of white matter FA (Fig. 1) and MD (Supplementary Fig. 1) detected an extended decrease in white matter FA and increase MD in the CP group in all cerebral lobes relative to controls. These differences were particularly prominent in the WM underlying the parietal lobe and, to a lesser extent, the temporal cortex. Differences in WM of the frontal and occipital lobes where less extended than those of the temporal and parietal lobes. Specifically, differences in frontal lobe were circumscribed in the anterior corona radiata, the genu of corpus callosum, the anterior part of the cingulate gyrus and the anterior part of the superior longitudinal fasciculus. Differences were further extended to the corticospinal tract, inferior and superior cerebellar peduncles and medial lemniscus.
Fig. 1.
Regions where fractional anisotropy was significantly lower in a sample of 33 subjects with dyskinetic cerebral palsy compared with 33 age and sex matched healthy controls. Results are shown at p < 0.05 corrected for multiple comparisons and overlaid on the group mean fractional anisotropy. RH: right hemisphere; LH: left hemisphere.
3.2.2. Relationship between IQ and white matter microstructure
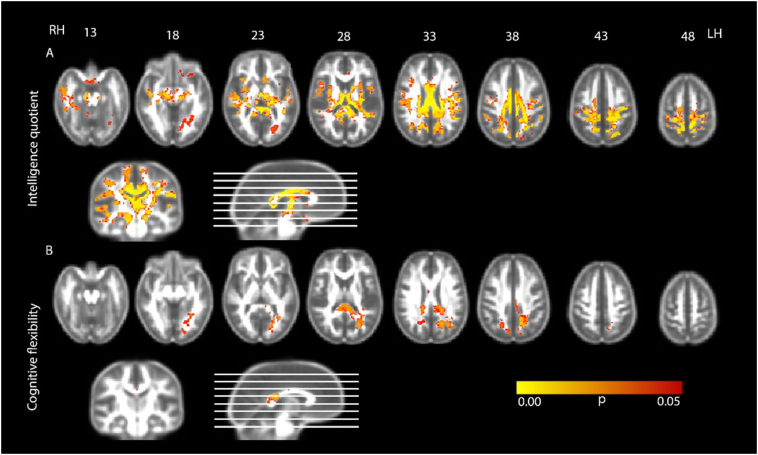
There was no significant correlation between IQ and WM microstructure in the control group. In the CP group, FA was significantly positively correlated with IQ in all cerebral lobes (Figs. 2a and 3; Table 3). Specifically, FA significantly correlated with IQ in six clusters. Fractional anisotropy and IQ correlated in almost the entire sagittal axis of the fornix and corpus callosum. A significant correlation was also observed bilaterally in the cerebral and cerebellar peduncles. Parts of the posterior thalamic radiation within the internal capsule and superior and posterior corona radiata were also significantly correlated with IQ, as were the external capsule, the cingulum, the superior longitudinal fasciculus and the superior fronto-occipital fasciculus. A significant correlation was further observed in the anterior limb of the internal capsule. Specifically, in the right hemisphere, FA was significantly correlated with IQ in the tapetum and sagittal stratum.
Fig. 2.
Regions where fractional anisotropy correlated A) positively with intelligence quotient (Raven's coloured progressive matrices) B) negatively with cognitive flexibility (Wisconsin card sorting test). Results are shown at p < 0.05, corrected for multiple comparisons, controlled for age and sex and overlaid on the group mean fractional anisotropy image. RH: right hemisphere; LH: left hemisphere.
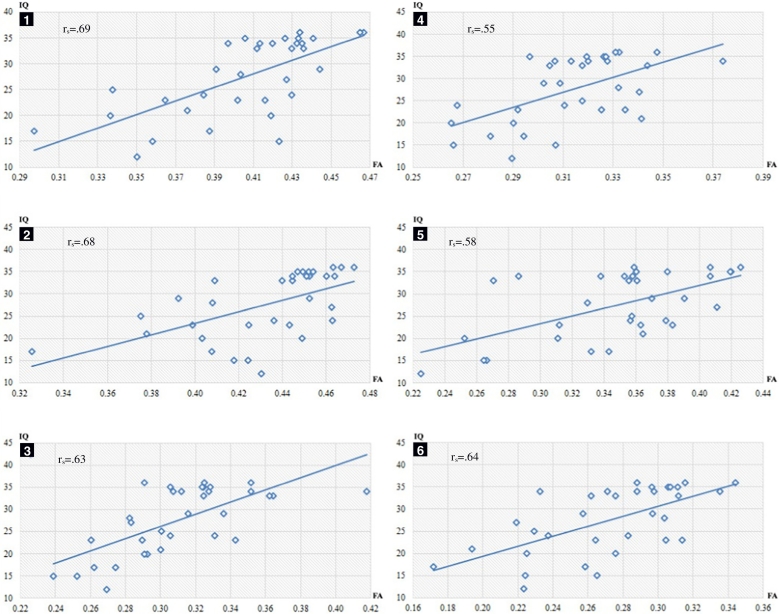
Fig. 3.
Scatterplot between IQ (intelligence quotient) (y-axis; raw score) and mean FA (fractional anisotropy) (x-axis) in significant clusters (p < 0.05). Clusters sorted by size (largest first) and labelled by the same numbers as Table 3.
Table 3.
Regions showing a positive correlation between fractional anisotropy and intelligence quotient in the cerebral palsy group.
| Cluster number | Cluster size (mm3) | Mean FA (SD) (CP/controls) | Anatomical regions |
||
|---|---|---|---|---|---|
| JHU white matter atlas | Harvard-Oxford cortical structural atlas | ||||
| 1 | 143,218 | 0.393 (0.099)/ 0.418 (0.105) |
MS | Genu, body and splenium of corpus callosum Column and body of fornix Middle cerebellar peduncle |
|
| B |
Fornix (cres) Cerebral peduncle Posterior thalamic radiation (include optic radiation) Posterior limb and retrolenticular part of internal capsule Superior and posterior corona radiata External capsule Cingulum (cingulate gyrus and hippocampus) Superior longitudinal fasciculus Superior fronto-occipital fasciculus (could be a part of anterior internal capsule) Anterior limb of internal capsule |
Insular cortex Superior,middle and inferior (pars opercularis) frontal gyrus Precentral gyrus Superior temporal gyrus (anterior and posterior division) Middle temporal gyrus (posterior division and temporo-occipital part) Postcentral gyrus Superior parietal lobe Supramarginal gyrus (anterior and posterior division) Cingulate gyrus (anterior and posterior division) Precuneous cortex Parahippocampal gyrus (anterior division) Supplementary motor cortex Central and parietal opercular cortex Planum polare Planum temporale Lateral occipital cortex (superior division) Angular gyrus Heschl's gyrus (includes H1 and H2) |
|||
| LH | Frontal operculum cortex | ||||
| RH | Tapetum Sagittal stratum (include inferior longitudinal fasciculus and inferior fronto-occipital fasciculus) |
Inferior frontal gyrus (pars triangularis) Cuneal cortex Parahippocampal gyrus (posterior and anterior division) Temporal fusiform cortex (posterior division) Temporal occipital fusiform cortex Inferior temporal gyrus (posterior division and temporo-occipital part) |
|||
| 2 | 3359 | 0.432 (0.087)/ 0.443(0.093) |
LH | Posterior thalamic radiation (include optic radiation) | Intracalcarine cortex Lingual gyrus Occipital fusiform gyrus Temporal occipital fusiform cortex |
| 3 | 1184 | 0.310 (0.036)/ 0.322 (0.037) |
B |
Frontal orbital cortex Subcallosal cortex |
|
| 4 | 890 | 0.314 (0.042)/ 0.315 (0.042) |
LH |
Frontal orbital cortex Subcallosal cortex |
|
| 5 | 147 | 0.349 (0.069)/ 0.352 (0.070) |
LH | Superior frontal gyrus | |
| 6 | 54 | 0.272 (0.024)/ 0.296 (0.028) |
LH | Lateral occipital cortex (superior division) | |
B: bilateral; CP: cerebral palsy; FA: fractional anisotropy; JHU: John Hopkins University; LH: left hemisphere; MS: medial structure; RH: right hemisphere; SD: standard deviation. Discrepancies between fractional anisotropy and mean diffusivity results are indicated as follows. Bold indicates regions that are significant with both fractional anisotropy and mean diffusivity. Italics indicate regions that only show the same result between fractional anisotropy and mean diffusivity in one hemisphere.
Intelligence quotient was also associated with WM microstructure close to several cortical regions: mainly the parietal cortex and the temporal cortex including the parahippocampal gyrus. These regions were not covered by the JHU atlas and were therefore labelled according to the Harvard-Oxford cortical structural atlas (Table 3). Four smaller significant clusters within the WM (not labelled with the JHU atlas) were located close to the frontal orbital cortex, superior frontal gyrus, subcallosal cortex and the superior division of the lateral occipital cortex.
The significant correlation between MD and IQ embraced fewer regions than that of FA and showed only one significant cluster. Specifically, FA was significantly correlated with IQ in the genu of corpus callosum, the middle cerebellar peduncle, the posterior thalamic radiation, the tapetum, sagital stratum and hippocampus section of the cingulum as defined by the JHU white matter atlas. Mean diffusivity was not related with these regions. Significant correlations between FA and IQ were also extended to more regions not covered by the JHU atlas than significant correlations between MD and IQ. The left uncinated fasciculus was the only region showing a significant correlation between MD and IQ but not FA and IQ. The relationship between MD and IQ is reported in Supplementary Table 1, and Supplementary Fig. 2, Supplementary Fig. 3. Differences between FA and MD results are indicated in Table 3 as well as Supplementary Table 1.
3.2.3. Relationship between executive function and white matter microstructure
There were no significant correlations between FA and executive function domains observed in the control group for any of the four analyses. Attentional control, goal setting and information processing did not correlate with WM microstructure in the CP group, however FA was significantly negatively correlated with WCST scores (which measure cognitive flexibility) in the CP group. These results indicate that higher FA is associated with better performance in cognitive flexibility (i.e. lower scores on the WCST). Specifically, there was a statistically significant negative correlation between cognitive flexibility (WCST scores) and FA in four clusters (Figs. 2b and 4; Table 4). The largest cluster was extended along the body and splenium of the corpus callosum including the left tapetum. For this cluster, FA was also correlated with WCST scores in bilateral WM regions in the posterior corona radiata. Fractional anisotropy of the retrolenticular part of the internal capsule, posterior thalamic radiation, the cingulate gyrus and the superior longitudinal fasciculus was further correlated with executive function in the left hemisphere. Regions where FA was correlated with cognitive flexibility were extended bilaterally to the WM below the posterior division of the cingulate gyrus according to the Harvard-Oxford cortical structural atlas. The relationship between WM microstructure and executive function was located in different regions in the left hemisphere near the precentral, postcentral, lingual, occipital fusiform and supramarginal gyrus. In this hemisphere, correlations were also found in WM near the intracalcarine, supracalcarine and cuneal cortex as well as the planum temporale and the inferior temporal gyrus. The second largest cluster was located in the right hemisphere, and executive function correlations were found in the WM near to the lateral occipital, superior parietal and precuneus cortex. For the two remaining smaller clusters, FA was correlated with WM close the superior parietal lobe and the anterior division of the cingulate gyrus.
Fig. 4.
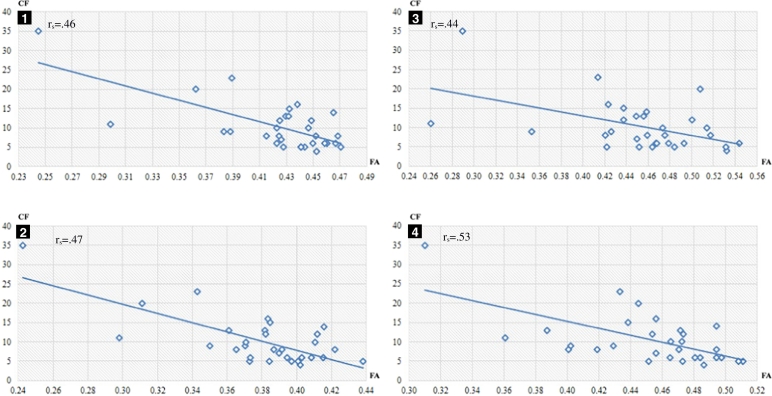
Scatterplot between CF (cognitive flexibility) (y-axis; raw score) and mean FA (fractional anisotropy) (x-axis) in significant clusters (p < 0.05). Clusters sorted by size (largest first) and labelled by the same numbers as Table 4.
Table 4.
Regions showing a negative correlation between fractional anisotropy and cognitive flexibility in the cerebral palsy group.
| Cluster number | Cluster size (mm3) | Mean FA (SD) (CP/controls) | Anatomical regions |
||
|---|---|---|---|---|---|
| JHU white matter atlas | Harvard-Oxford cortical structural atlas | ||||
| 1 | 20,951 | 0.450 (0.104)/ 0.479 (0.110) |
MS | Body and splenium of corpus callosum | |
| B | Posterior corona radiata | Cingulate gyrus (posterior division) | |||
| LH | Tapetum Retrolenticular part of internal capsule Posterior thalamic radiation (include optic radiation) Cingulum (cingulate gyrus) Superior longitudinal fasciculus |
Precentral gyrus Postcentral gyrus Lingual gyrus Occipital fusiform gyrus Supramarginal gyrus (anterior and posterior division) Intracalcarine cortex Supracalcarine cortex Cuneal cortex Planum temporale Inferior temporal gyrus (temporo-occipital part) |
|||
| 2 | 245 | 0.379 (0.070)/ 0.392 (0.074) |
RH |
Lateral occipital cortex (superior division) Superior parietal lobe Precuneous cortex |
|
| 3 | 69 | 0.379 (0.070)/ 0.392 (0.074) |
RH | Superior parietal lobe | |
| 4 | 54 | 0.453 (0.080)/ 0.474 (0.085) |
RH | Cingulate gyrus (anterior division) | |
B: bilateral; CP: cerebral palsy; FA: fractional anisotropy; JHU: John Hopkins University; LH: left hemisphere; MS: medial structure; RH: right hemisphere; SD: standard deviation. Discrepancies between fractional anisotropy and mean diffusivity results are indicated as follows. Bold indicates regions that are significant with both fractional anisotropy and mean diffusivity. Italics indicate regions that only show the same result between fractional anisotropy and mean diffusivity in one hemisphere.
The significant correlations between MD and executive function embraced smaller volume than those of FA and were mainly located in the left hemisphere. While MD was not significantly related with executive function in some of the previously mentioned regions (body of corpus callosum, tapetum, retrolenticular part of internal capsule, posterior thalamic radiation and the cingulum), it was related in the superior corona radiata and the superior fronto-occipital fasciculus (according to the JHU white matter atlas). The relationship between MD and executive function is reported in Supplementary Table 2, and Supplementary Fig. 2, Supplementary Fig. 4. Discrepancies between FA and MD results are indicated in Table 4 as well as Supplementary Table 2.
4. Discussion
To our knowledge, this is the first study to examine the association between WM microstructure and executive function in a relatively large sample of people with dyskinetic CP (n = 33) compared to a group of age and sex matched healthy controls. Although dyskinetic CP has been classically characterized by deep grey matter injury (playing an important role in the specific movement disorder), this study shows that WM microstructure is also impaired in all cerebral lobes in both sensori-motor and non-motor-related regions. These impairments may explain other comorbidities such as cognitive difficulties in dyskinetic CP. It is noteworthy that the loss in the integrity of the WM in dyskinetic CP is predominantly underlying the parietal and, to a lesser extent, the temporal cortex. Our larger sample confirmed the early observations of Yoshida et al. (2013) in a sample of seven children with dyskinetic CP. Yoshida et al. (2013) performed analysis of 205 atlas-defined regions, and reported that extended WM changes were present along the main four brain lobes.
Regarding our investigation of the correlation between cognitive function and WM microstructure, we cannot compare findings in CP against our control group because none of the cognitive functions assessed were significantly related with FA in the control group. A previous study exploring the association between FA and perseverative responses of the WCST in typically developing subjects did not find any significant correlation (Ohtani et al., 2017), in agreement with our results. To understand the neuroimaging results it is necessary to keep in mind the cognitive performance of our sample. We hypothesise that the restricted range of neuropsychological scores prevented finding significant associations between brain microstructure and function in the control group, unlike the CP group scores with a wider spread of neuropsychological scores, for whom significant correlations were revealed. The higher variability in the CP group in almost all neuropsychological scores, as well as their differential relationship with the WM microstructure, are signs that the biological basis of cognition in dyskinetic CP could be different to the general population. While findings in CP cannot be compared against the control group, interesting conclusions may be drawn from the associations observed in people with dyskinetic CP.
Intelligence quotient was related to WM integrity in the majority of regions that also showed a decrease in FA relative to the control group. These results for IQ are consistent with the literature reporting the importance of undisrupted information transfer in cortico-cortical long association fibres in intelligence (Deary et al., 2010) such as the superior longitudinal fasciculus, sagittal stratum, superior fronto-occipital fasciculus or the cingulum. Our results are also consistent with a previous study in an overlapping cohort of dyskinetic CP participants reporting a correlation between RCPM and the WM volume in the superior longitudinal fasciculus, the internal capsule and the cingulum using WM voxel based morphometry analysis of T1-weighted images (Ballester-Plané et al., 2016). Interestingly, a previous study showed specific reduction in long-range connectivity in severe versus moderate CP (Englander et al., 2013). Although the study by Englander et al. (2013) did not focus on dyskinetic CP and did not include participants with GMFCS level V, all participants were diagnosed with bilateral CP and included some participants with dyskinetic features. Other regions that showed significant associations with IQ in our study, such as the corpus callosum and the fornix are also involved in non-motor functions (Fling et al., 2016, Leech and Sharp, 2014). Intelligence quotient was further associated with WM microstructure in some regions consistent with the fronto-striatal circuit, such as the anterior limb of the internal capsule, which is involved in cognition (Oberlin et al., 2016). This result is not surprising as the basal ganglia are involved in both executive function and IQ as are crucial elements in the circuits that confer human reasoning (Leisman et al., 2014). It is also noteworthy that we found that regions of association between FA and IQ extended to the superior section of the tracts close to the frontal cortex which have been reported to be involved in general cognitive functioning (Ohtani et al., 2017). Other WM regions close to the parietal and occipital cortex and involved in perception also showed correlations between FA and IQ. The association between WM microstructure and IQ in the parahippocampal and temporal cortex could be due to injury in the hippocampus in some participants of our CP group, as has been previously reported in infants asphyxiated at term (De Haan et al., 2006). This form of injury also seems to be associated with damage to the basal ganglia and thalamus. Thus, the relationship between IQ and FA in these regions could reflect a degree of covariance between the level of asphyxia and IQ.
While some regions that show a significant relationship between brain microstructure and IQ play a crucial role in the general cognitive functioning in dyskinetic CP, others might be underlying the motor impairment which is highly associated with the intellectual ability (Gabis et al., 2016). The FA was also related with IQ in specific WM regions associated with sensori-motor skills. These cortico-subcortical pathways, including the superior and posterior corona radiata and their projections to the precentral and postcentral gyrus, or the posterior thalamic radiation, may reflect a degree of covariance between motor function and IQ, as previously suggested (Smits-Engelsman and Hill, 2012). The association was further extended to the cerebral and cerebellar peduncles.
It is important to highlight the main differences between FA correlations with IQ and the FA differences between the CP and control group. While clusters resulting from the contrast between the CP and control group were extended to the left inferior temporal gyrus and to the inferior parts of the cerebrum, the IQ were not associated with FA in these brain regions. Overall, reduced FA correlated with lower IQ in several cortico-cortical and cortico-subcortical regions. This is in accordance with the general consensus that intelligence does not reside in a single, narrowly circumscribed brain region but requires a widespread network (Deary et al., 2010).
Cognitive flexibility measured by the WCST was sensitive to the WM integrity in our CP sample. Our results are consistent with previous studies in other CP samples showing that WM features are associated with executive function (Scheck et al., 2015) and specifically with WCST (Skranes et al., 2008). In particular, a study investigating quantitatively the relationship between executive function and connectivity in unilateral CP reports the involvement of the anterior cingulate (Scheck et al., 2015) while in dyskinetic CP we mainly found significant associations in the WM of the middle and posteriors parts of the cingulum. This difference may be due to the fact that the study of Scheck et al. (2015) focused on unilateral CP while our participants have dyskinetic CP.
Cognitive flexibility was associated with FA in regions known to contain connections with the frontal lobe such as the cingulate gyrus and the superior longitudinal fasciculus in the left hemisphere but also in regions not directly connected with the frontal lobe such as the posterior corpus callosum and superior and posterior corona radiata, the retrolenticular part of internal capsule and the posterior thalamic radiation. This is not surprising because WM disturbances in non-frontal regions can affect the connectivity of the entire brain, impairing communication between frontal lobe and other brain regions, and thereby causing executive function difficulties (Bettcher et al., 2016). Cognitive flexibility did not correlate directly with FA in the anterior part of the frontal lobe. The most anterior location where we found a correlation between WM microstructure and executive function was located in the WM near the precentral gyrus. Our results are located in more posterior regions compared with those reported by Ohtani et al. (2017) in a typically developing sample. Specifically, higher FA values in the right anterior middle orbital frontal cortex and the rostral anterior cingulate cortex were associated with more correct responses on the WCST in Ohtani et al. (2017). In agreement with Ohtani et al. (2017) another study reported that WM hyperintensities in the prefrontal region were independently associated with age-related increases in perseverative errors on the WCST (Gunning-Dixon and Raz, 2003). These results also seem to be contradictory to our findings that did not show such relationships in any prefrontal region. Impairments in executive function have been reported to be due to cortical-subcortical damage even when the frontal lobe is not directly damaged (Little et al., 2010). Accordingly, a study using a different measure of cognitive flexibility suggested the basal ganglia and the fronto-striato-thalamic circuit play crucial role in cognitive flexibility in healthy adults (Van Schouwenburg et al., 2014). Unexpectedly, there was neither significant relationship between FA and executive function near the fronto-striatal pathways in our study. This may be due to the fact that fronto-striatal circuits interact with other complex, cortical-subcortical circuits relevant to behaviour, such as the visual association regions of the temporal cortex, the hippocampus and the amygdala (Chudasama and Robbins, 2006) which could play an important role in the WCST performance in our sample. Interestingly, a study using another measure of cognitive flexibility found that higher FA was associated with better cognitive flexibility in posterior brain regions in children (Treit et al., 2014). Further research is needed to elucidate whether the negative finding in anterior regions might be a sign of neuroplastic reorganization.
The relationship between FA and cognitive flexibility was more predominant on the left hemisphere. The laterality observed in cognitive flexibility is not consistent with a previous study in unilateral CP assessing the four executive function domains (Bodimeade et al., 2013) but it was consistent with a previous study of cortical thickness in dyskinetic CP including 28 of the participants included in the present study (Laporta-Hoyos et al., 2017). This interhemispheric difference observed with the WCST has also been previously described in other neurological conditions (Jodzio et al., 2016).
All mean diffusivity results were relatively similar to FA. Even so, it is important to point out that FA and MD results are not completely coincident. These slight differences are not surprising as FA and MD are different measures of microstructure and previous studies focused on CP also found few differences between FA and MD results (Scheck et al., 2015, Yoshida et al., 2011). While high FA index reflects preserved WM and coherence of diffusion, MD is a measure of the total diffusion within a voxel and high values indicates tissue alteration (Le Bihan et al., 2001; Mori & Zhang, 2006). Overall, MD results for correlations seem to be less extended and the correlation between MD and executive function is more left sided than the correlation between FA and executive function. In the light of these results, it is important to take into account that although DTI does not allow examination of the cytoarchitecture of the WM tissue, increased FA in crossing-fiber regions could be reflecting a decrement in FA in a contributing fiber bundle (Douaud et al., 2011; Groeschel et al., 2014).
There are some potential limitations to our study. First, the performance observed in our sample cannot be generalized to the broad population of dyskinetic CP because we have included only subjects with adequate comprehension. It is also noteworthy that the study of cognitive function is only possible in this subsample of dyskinetic CP who had a minimum comprehension level. Second, the MRI requires to hold still or agreement to be sedated, therefore some participants satisfying the inclusion criteria were not able to be included in this study. Further participants were removed because of registration errors, because the method can only be used when brain pathology is not substantial. Third, the control group presents an above average cognitive functioning indicating that a larger sample size would be more representative of the general population. Fourth, although the age range is very wide, age-corrected raw scores had to be used because not all tests provide standardized scores. A replication of the analyses using a narrower age band would help to strengthen the present results. Fifth, the speed of response is a potential confound in some tasks such as information processing and attentional control because participants are encouraged to respond as fast as possible which may cause additional stress for the CP participants. In the attentional control task, it is further noteworthy that motor impairment might actually overestimate the performance of CP participants on “stop trials” (25% of the total trials) as motor slowness might preclude inhibition errors. Even so, reaction time scores are not taken into account for the analyses and SST is a computerized test with a low motor component because the stimulus does not disappear from the screen until the participant responds. Sixth, although the sample size may be considered large taking into account the characteristics of the sample and the previous studies, it is small in terms of statistical power.
5. Conclusion
Despite the difficulties in the assessment, brain structure and cognition can be studied in dyskinetic CP. It was demonstrated that dyskinetic CP was associated with widespread WM microstructural changes in sensori-motor and non-motor areas. Significant FA differences between dyskinetic CP and healthy controls were widespread in all lobes but are more extended near the parietal cortex. Lower IQ was correlated with widespread damage, including the superior section of tracts showing a reduced FA. While some of these regions play a crucial role in the IQ in dyskinetic CP and should be targets for future research, others might be underlying the motor impairment which is highly associated with the intellectual ability. Regarding executive function domains, their correlation with white matter FA was only significant for cognitive flexibility. Preserved cognitive flexibility was associated with regions known to contain cortico-cortical pathways, some of which are highly connected with the frontal lobe, and cortico-subcortical pathways not directly connected with frontal lobe. Although basal ganglia and thalamus injury are associated with dyskinetic CP, executive function seems not to be specifically related to the WM of the fronto-striatal circuit as expected. Further research is needed to elucidate whether the negative finding in this region might be a sign of neuroplastic reorganization.
The following are the supplementary data related to this article.
Regions showing a negative correlation between mean diffusivity and intelligence quotient in the cerebral palsy group.
Regions showing a positive correlation between mean diffusivity and cognitive flexibility in the cerebral palsy group.
Regions where mean diffusivity was significantly higher in a sample of 33 subjects with dyskinetic cerebral palsy compared with 33 age and sex matched healthy controls. Results are shown at p < 0.05 corrected for multiple comparisons and overlaid on the group mean diffusivity image. RH: right hemisphere; LH: left hemisphere.
Regions where mean diffusivity correlated A) negatively with intelligence quotient (Raven's coloured progressive matrices) B) positively with cognitive flexibility (Wisconsin card sorting test). Results are shown at p < 0.05, corrected for multiple comparisons, controlled for age and sex and overlaid on the group mean fractional anisotropy image. RH: right hemisphere; LH: left hemisphere.
Scatterplot between IQ (intelligence quotient) (y-axis; raw score) and mean MD (mean diffusivity) (x-axis) in significant clusters (p < 0.05).
Scatterplot between CF (cognitive flexibility) (y-axis; raw score) and mean MD (mean diffusivity) (x-axis) in significant clusters (p < 0.05). Clusters sorted by size (largest first) and labelled by the same numbers as Supplementary Table 2.
Supplementary material 1
Supplementary material 2
Supplementary material 3
Fundings
This work was supported by the Ministerio de Ciencia e Innovación (grant code PSI2011/24386), by the Ministerio de Economía y Competitividad (PSI2016-75979-R AEI/FEDER, UE) and by Generalitat de Catalunya (2014SGR98). Roslyn Boyd is supported by a NHMRC Research Fellowship. Olga Laporta received a research grant from Ministerio de educación, cultura y deporte of the government of Spain (grant code FPU13/06435) and an Endeavour research Fellowship by the Australian Government (grant code 5240).
Acknowledgments
The authors wish to thank all participants and their families for their collaboration. We would also like to thank the association ASPACE and the Vigatans and ASDI centers for providing patients enrolled in this study, as well as UTAC service of the Faculty of Psychology at the University of Barcelona for their guidance in adapting tests. We further appreciate the Endeavour Fellowship provided by the Australian Government to Olga Laporta to undertake training on data analysis at the Queensland Cerebral Palsy and Rehabilitation Research Centre at the University of Queensland; the Australian e-Health Research Centre at Commonwealth Scientific and Industrial Research Organisation.
References
- American Psychiatric Association . American Psychiatric Association; Washington: 2013. Diagnostic and Statistical Manual of Mental Disorders: DSM-5. [Google Scholar]
- Anderson V. Taylor & Francis; New York: 2008. Executive Functions and the Frontal Lobes: A Lifespan Perspective. [Google Scholar]
- Andersson J.L.R., Sotiropoulos S.N. An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. NeuroImage. 2015;125:1063–1078. doi: 10.1016/j.neuroimage.2015.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avants B.B., Tustison N.J., Song G., Cook P.A., Klein A., Gee C. Vol. 54. 2012. NIH Public Access; pp. 2033–2044. [Google Scholar]
- Ballester-Plané J., Laporta-hoyos O., Macaya A., Póo P., Meléndez-plumed M., Vázquez E., Delgado I., Narberhaus A., Toro-tamargo E., Eugenia M., Tenorio V., Segarra D., Pueyo R. Measuring intellectual ability in cerebral palsy: the comparison of three tests and their neuroimaging correlates. Res. Dev. Disabil. 2016;56:83–98. doi: 10.1016/j.ridd.2016.04.009. [DOI] [PubMed] [Google Scholar]
- Basser P.J., Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J. Magn. Reson. B. 1996;111(3):209–219. doi: 10.1006/jmrb.1996.0086. [DOI] [PubMed] [Google Scholar]
- Bax M., Tydeman C., Flodmark O. Clinical and MRI correlates of cerebral palsy: the European cerebral palsy study. JAMA. 2006;296:1602–1608. doi: 10.1001/jama.296.13.1602. [DOI] [PubMed] [Google Scholar]
- Bettcher B.M., Mungas Dan, Patel N., Elofson J., Dutt S., Wynn M., Watson C.L., Stephens M., Walsh C.M., Kramer J.H. Neuroanatomical substrates of executive functions: beyond prefrontal structures. Neuropsychologia. 2016;85:100–109. doi: 10.1016/j.neuropsychologia.2016.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodimeade H.L., Whittingham K., Lloyd O., Boyd R.N. Executive function in children and adolescents with unilateral cerebral palsy. Dev. Med. Child Neurol. 2013;55:926–933. doi: 10.1111/dmcn.12195. [DOI] [PubMed] [Google Scholar]
- Bottcher L. Children with spastic cerebral palsy, their cognitive functioning, and social participation: a review. Child Neuropsychol. 2010;16:209–228. doi: 10.1080/09297040903559630. [DOI] [PubMed] [Google Scholar]
- Cambridge Cognition . Cambridge Cognition; Cambridge: 1999. Limited Cambridge Neuropsychological Test Automated Battery. [Google Scholar]
- Christ S.E., White D.A., Brunstrom J.E., Abrams R.A. Inhibitory control following perinatal brain injury. Neuropsychology. 2003;17:171–178. [PubMed] [Google Scholar]
- Chudasama Y., Robbins T.W. Functions of frontostriatal systems in cognition: comparative neuropsychopharmacological studies in rats, monkeys and humans. Biol. Psychol. 2006;73:19–38. doi: 10.1016/j.biopsycho.2006.01.005. [DOI] [PubMed] [Google Scholar]
- De Haan M., Wyatt J.S., Roth S., Vargha-Khadem F., Gadian D., Mishkin M. Brain and cognitive-behavioural development after asphyxia at term birth. Dev. Sci. 2006;9:350–358. doi: 10.1111/j.1467-7687.2006.00499.x. [DOI] [PubMed] [Google Scholar]
- Deary I.J., Penke L., Johnson W. The neuroscience of human intelligence differences. Nat. Rev. Neurosci. 2010;11:201–211. doi: 10.1038/nrn2793. [DOI] [PubMed] [Google Scholar]
- Englander Z.a., Pizoli C.E., Batrachenko A., Sun J., Worley G., Mikati M.a., Kurtzberg J., Song A.W. Diffuse reduction of white matter connectivity in cerebral palsy with specific vulnerability of long range fiber tracts. NeuroImage Clin. 2013;2:440–447. doi: 10.1016/j.nicl.2013.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fling B.W., Dale M.L., Curtze C., Smulders K., Nutt J.G., Horak F.B. Associations between mobility, cognition and callosal integrity in people with parkinsonism. NeuroImage Clin. 2016;11:415–422. doi: 10.1016/j.nicl.2016.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonslow B.R., Stein B.D., Webb K.J., Xu T., Choi J., Kyu S., Iii J.R.Y. Vol. 10. 2013. NIH Public Access; pp. 54–56. [Google Scholar]
- Gabis L.V., Tsubary N.M., Leon O., Ashkenasi A., Shefer S. Vol. 30. 2016. Assessment of Abilities and Comorbidities in Children With Cerebral Palsy; pp. 1640–1645. [DOI] [PubMed] [Google Scholar]
- Gramunt-Fombuena N., Pen J. 2009. Spanish Multicenter Normative Studies (NEURONORMA Project): Norms for Verbal Fluency Tests. [DOI] [PubMed] [Google Scholar]
- Gunning-Dixon F.M., Raz N. Neuroanatomical correlates of selected executive functions in middle-aged and older adults: a prospective MRI study. Neuropsychologia. 2003;41:1929–1941. doi: 10.1016/s0028-3932(03)00129-5. [DOI] [PubMed] [Google Scholar]
- Himmelmann K., Uvebrant P. Function and neuroimaging in cerebral palsy: a population-based study. Dev. Med. Child Neurol. 2011;53:516–521. doi: 10.1111/j.1469-8749.2011.03932.x. [DOI] [PubMed] [Google Scholar]
- Himmelmann K., Hagberg G., Wiklund L.M., Eek M.N., Uvebrant P. Dyskinetic cerebral palsy: a population-based study of children born between 1991 and 1998. Dev. Med. Child Neurol. 2007;49:246–251. doi: 10.1111/j.1469-8749.2007.00246.x. [DOI] [PubMed] [Google Scholar]
- Himmelmann K., McManus V., Hagberg G., Uvebrant P., Krägeloh-Mann I., Cans C., Krageloh-Mann I., SCPE Collaboration Dyskinetic cerebral palsy in Europe: trends in prevalence and severity. Arch. Dis. Child. 2009;94:921–926. doi: 10.1136/adc.2008.144014. [DOI] [PubMed] [Google Scholar]
- Hua K., Zhang J., Wakana S., Jiang H., Li X., Reich D.S., Calabresi P.A., Pekar J.J., van Zijl P.C.M., Mori S. Tract probability maps in stereotaxic spaces: analyses of white matter anatomy and tract-specific quantification. NeuroImage. 2008;39:336–347. doi: 10.1016/j.neuroimage.2007.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenks K.M., de Moor J., van Lieshout E.C. Arithmetic difficulties in children with cerebral palsy are related to executive function and working memory. J. Child Psychol. Psychiatry. 2009;50:824–833. doi: 10.1111/j.1469-7610.2008.02031.x. [DOI] [PubMed] [Google Scholar]
- Jodzio K., Biechowska D., Jodzio K., Biechowska D. 2016. Wisconsin Card Sorting Test as a Measure of Executive Function Impairments in Stroke Patients; p. 4282. [DOI] [PubMed] [Google Scholar]
- Jung R.E., Haier R.J. The Parieto-Frontal Integration Theory (P-FIT) of intelligence: converging neuroimaging evidence. Behav. Brain Sci. 2007;30 doi: 10.1017/S0140525X07001185. 10.1017/S0140525X07001185 (135-154-187) [DOI] [PubMed] [Google Scholar]
- Kolk A., Talvik T. Cognitive outcome of children with early-onset hemiparesis. J. Child Neurol. 2000;15:581–587. doi: 10.1177/088307380001500903. [DOI] [PubMed] [Google Scholar]
- Kongs S.K., Thompson L.L., Iverson G.L., Heaton R.K. Psychological Assessment Resources Inc. FL; Lutz: 2000. Wisconsin card sortin test – 64 card version. [Google Scholar]
- Krageloh-Mann I., Cans C. Cerebral palsy update. Brain Dev. 2009;31:537–544. doi: 10.1016/j.braindev.2009.03.009. [DOI] [PubMed] [Google Scholar]
- Laporta-Hoyos O., Ballester-Plané J., Póo P., Macaya A., Meléndez-Plumed M., Vázquez E., Delgado I., Zubiaurre-Elorza L., Botellero V.L., Narberhaus A., Toro-Tamargo E., Segarra D., Pueyo R. Proxy-reported quality of life in adolescents and adults with dyskinetic cerebral palsy is associated with executive functions and cortical thickness. Qual. Life Res. 2017;26:1209–1222. doi: 10.1007/s11136-016-1433-0. [DOI] [PubMed] [Google Scholar]
- Le Bihan D., Mangin J.F., Poupon C., Clark C.A., Pappata S., Molko N., Chabriat H. Diffusion tensor imaging: concepts and applications. J. Magn. Reson. Imaging. 2001;13:534–546. doi: 10.1002/jmri.1076. [DOI] [PubMed] [Google Scholar]
- Leech R., Sharp D.J. The role of the posterior cingulate cortex in cognition and disease. Brain. 2014;137:12–32. doi: 10.1093/brain/awt162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leisman G., Braun-Benjamin O., Melillo R. Cognitive-motor interactions of the basal ganglia in development. Front. Syst. Neurosci. 2014;8:16. doi: 10.3389/fnsys.2014.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little D.M., Kraus M.F., Joseph J., Geary E.K., Susmaras T., Zhou X.J., Pliskin N., Gorelick P.B. Thalamic integrity underlies executive dysfunction in traumatic brain injury. Neurology. 2010;74:558–564. doi: 10.1212/WNL.0b013e3181cff5d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Measso G., Zappalà G., Cavarzeran F., Crook T.H., Romani L., Pirozzolo F.J.…Lebowitz D. Raven's colored progressive matrices: a normative study of a random sample of healthy adults. Acta Neurol. Scand. 1993;88:70–74. doi: 10.1111/j.1600-0404.1993.tb04190.x. [DOI] [PubMed] [Google Scholar]
- Nichols T.E., Holmes A.P. Nonparametric permutation tests for {PET} functional neuroimaging experiments: a primer with examples. Hum. Brain Mapp. 2001;15:1–25. doi: 10.1002/hbm.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak I., Hines M., Goldsmith S., Barclay R. Clinical prognostic messages from a systematic review on cerebral palsy. Pediatrics. 2012;130:e1285–e1312. doi: 10.1542/peds.2012-0924. [DOI] [PubMed] [Google Scholar]
- Oberlin L.E., Verstynen T.D., Burzynska A.Z., Voss M.W., Prakash R.S., Chaddock-Heyman L., Wong C., Fanning J., Awick E., Gothe N., Phillips S.M., Mailey E., Ehlers D., Olson E., Wojcicki T., McAuley E., Kramer A.F., Erickson K.I. White matter microstructure mediates the relationship between cardiorespiratory fitness and spatial working memory in older adults. NeuroImage. 2016;131:91–101. doi: 10.1016/j.neuroimage.2015.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtani T., Nestor P.G., Bouix S., Newell D., Melonakos E.D., McCarley R.W., Shenton M.E., Kubicki M. Exploring the neural substrates of attentional control and human intelligence: diffusion tensor imaging of prefrontal white matter tractography in healthy cognition. Neuroscience. 2017;341:52–60. doi: 10.1016/j.neuroscience.2016.11.002. [DOI] [PubMed] [Google Scholar]
- Pueyo R., Junque C., Vendrell P., Narberhaus A., Segarra D. Neuropsychologic impairment in bilateral cerebral palsy. Pediatr. Neurol. 2009;40:19–26. doi: 10.1016/j.pediatrneurol.2008.08.003. [DOI] [PubMed] [Google Scholar]
- Rai Y., Chaturvedi S., Paliwal V.K., Goyal P., Chourasia A., Singh Rathore R.K., Yadav A., Pandey C.M., Lalla R.S., Garg R.K., Gupta R.K. DTI correlates of cognition in term children with spastic diplegic cerebral palsy. Eur. J. Paediatr. Neurol. 2013;17:294–301. doi: 10.1016/j.ejpn.2012.11.005. [DOI] [PubMed] [Google Scholar]
- Raven J.C., Court J.H., Seisdedos Cubero N. TEA Ediciones; Madrid: 2001. Raven Matrices Progresivas Escalas: CPM Color, SPM General, APM Superior. [Google Scholar]
- Reid L.B., Cunnington R., Boyd R.N., Rose S.E. Surface-based fMRI-driven diffusion tractography in the presence of significant brain pathology: a study linking structure and function in cerebral palsy. PLoS One. 2016;11:1–25. doi: 10.1371/journal.pone.0159540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riva D., Franceschetti S., Erbetta A., Baranello G., Esposito S., Bulgheroni S. Congenital brain damage: cognitive development correlates with lesion and electroencephalographic features. J. Child Neurol. 2012;28:446–454. doi: 10.1177/0883073812447684. [DOI] [PubMed] [Google Scholar]
- Rosenbaum P., Paneth N., Leviton A., Goldstein M., Bax M., Damiano D., Dan B., Jacobsson B. A report: the definition and classification of cerebral palsy April 2006. Dev. Med. Child Neurol. Suppl. 2007;109:8–14. [PubMed] [Google Scholar]
- Scheck S.M., Boyd R.N., Rose S.E. New insights into the pathology of white matter tracts in cerebral palsy from diffusion magnetic resonance imaging: a systematic review. Dev. Med. Child Neurol. 2012;54:684–696. doi: 10.1111/j.1469-8749.2012.04332.x. [DOI] [PubMed] [Google Scholar]
- Scheck S.M., Pannek K., Raffelt D.A., Fiori S., Boyd R.N., Rose S.E. Structural connectivity of the anterior cingulate in children with unilateral cerebral palsy due to white matter lesions. NeuroImage Clin. 2015;9:498–505. doi: 10.1016/j.nicl.2015.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz C.G., Reid R.I., Gunter J.L., Senjem M.L., Przybelski S.A., Zuk S.M., Whitwell J.L., Vemuri P., Josephs K.A., Kantarci K., Thompson P.M., Petersen R.C., Jack C.R., Jr., ADNI Improved DTI registration allows voxel-based analysis that outperforms Tract-Based Spatial Statistics. NeuroImage. 2013;16:387–393. doi: 10.1016/j.neuroimage.2014.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigurdardottir S., Eiriksdottir A., Gunnarsdottir E., Meintema M., Arnadottir U., Vik T. Cognitive profile in young Icelandic children with cerebral palsy. Dev. Med. Child Neurol. 2008;50:357–362. doi: 10.1111/j.1469-8749.2008.02046.x. [DOI] [PubMed] [Google Scholar]
- Skranes J., Evensen K.I., Lohaugen G.C., Martinussen M., Kulseng S., Myhr G. Abnormal cerebral MRI findings and neuroimpairments in very low birth weight (VLBW) adolescents. Eur. J. Paediatr. Neurol. 2008;12(4):273–283. doi: 10.1016/j.ejpn.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Smith S.M. Fast robust automated brain extraction. Hum. Brain Mapp. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S.M., Nichols T.E. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. NeuroImage. 2009;44:83–98. doi: 10.1016/j.neuroimage.2008.03.061. [DOI] [PubMed] [Google Scholar]
- Smith S.M., Jenkinson M., Johansen-Berg H., Rueckert D., Nichols T.E., Mackay C.E., Watkins K.E., Ciccarelli O., Cader M.Z., Matthews P.M., Behrens T.E.J. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. NeuroImage. 2006;31:1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Smits-Engelsman B., Hill E.L. The relationship between motor coordination and intelligence across the IQ range. Pediatrics. 2012;130:e950–e956. doi: 10.1542/peds.2011-3712. [DOI] [PubMed] [Google Scholar]
- Straub K., Obrzut J.E. Effects of cerebral palsy on neuropsychological function. J. Dev. Phys. Disabil. 2009;21:153–157. [Google Scholar]
- Strauss E., Spreen O., Sherman E.M.S. 2006. A Compendium of Neuropsychological Tests; Administration, Norms, and Commentary. [Google Scholar]
- Surveillance of Cerebral Palsy in Europe Surveillance of cerebral palsy in Europe: a collaboration of cerebral palsy surveys and registers. Dev. Med. Child Neurol. 2000;42:816–824. doi: 10.1017/s0012162200001511. [DOI] [PubMed] [Google Scholar]
- Toronto A.S. Northwestern University Press; Evenston, IL: 1973. Screening Test of Spanish Grammar. [Google Scholar]
- Towsley K., Shevell M.I., Dagenais L., REPACQ Consortium Population-based study of neuroimaging findings in children with cerebral palsy. Eur. J. Paediatr. Neurol. 2011;15:29–35. doi: 10.1016/j.ejpn.2010.07.005. [DOI] [PubMed] [Google Scholar]
- Treit S., Chen Z., Rasmussen C., Beaulieu C. White matter correlates of cognitive inhibition during development: a diffusion tensor imaging study. Neuroscience. 2014;276:87–97. doi: 10.1016/j.neuroscience.2013.12.019. [DOI] [PubMed] [Google Scholar]
- Van Schouwenburg M.R., Onnink A.M.H., ter Huurne N., Kan C.C., Zwiers M.P., Hoogman M., Franke B., Buitelaar J.K., Cools R. Cognitive flexibility depends on white matter microstructure of the basal ganglia. Neuropsychologia. 2014;53:171–177. doi: 10.1016/j.neuropsychologia.2013.11.015. [DOI] [PubMed] [Google Scholar]
- Whittingham K., Bodimeade H.L., Lloyd O., Boyd R.N. Everyday psychological functioning in children with unilateral cerebral palsy: does executive functioning play a role? Dev. Med. Child Neurol. 2014;56:572–579. doi: 10.1111/dmcn.12374. [DOI] [PubMed] [Google Scholar]
- Yoshida S., Hayakawa K., Oishi K., Mori S., Kanda T., Yamori Y., Yoshida N., Hirota H., Iwami M., Okano S., Matsushita H., Imaging B.D. Athetotic and spastic cerebral palsy: anatomic characterization based on diffusion-tensor imaging. Radiology. 2011;260:511–520. doi: 10.1148/radiol.11101783. [DOI] [PubMed] [Google Scholar]
- Yoshida S., Faria A.V., Oishi K., Kanda T., Yamori Y., Yoshida N.…Mori S. Anatomical characterization of athetotic and spastic cerebral palsy using an atlas-based analysis. J. Magn. Reson. Imaging. 2013;29:997–1003. doi: 10.1002/jmri.23931. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Regions showing a negative correlation between mean diffusivity and intelligence quotient in the cerebral palsy group.
Regions showing a positive correlation between mean diffusivity and cognitive flexibility in the cerebral palsy group.
Regions where mean diffusivity was significantly higher in a sample of 33 subjects with dyskinetic cerebral palsy compared with 33 age and sex matched healthy controls. Results are shown at p < 0.05 corrected for multiple comparisons and overlaid on the group mean diffusivity image. RH: right hemisphere; LH: left hemisphere.
Regions where mean diffusivity correlated A) negatively with intelligence quotient (Raven's coloured progressive matrices) B) positively with cognitive flexibility (Wisconsin card sorting test). Results are shown at p < 0.05, corrected for multiple comparisons, controlled for age and sex and overlaid on the group mean fractional anisotropy image. RH: right hemisphere; LH: left hemisphere.
Scatterplot between IQ (intelligence quotient) (y-axis; raw score) and mean MD (mean diffusivity) (x-axis) in significant clusters (p < 0.05).
Scatterplot between CF (cognitive flexibility) (y-axis; raw score) and mean MD (mean diffusivity) (x-axis) in significant clusters (p < 0.05). Clusters sorted by size (largest first) and labelled by the same numbers as Supplementary Table 2.
Supplementary material 1
Supplementary material 2
Supplementary material 3