Table 1.
Scope of Multicomponent Carboamination of Tethered Enynes with PhNNPha
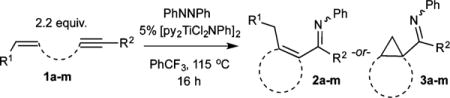
| |||
|---|---|---|---|
|
| |||
| Substrate | % Isolated Yieldb (2:3) | 1H NMR % Yield (2:3) | |
| 1a |
|
50 (>99:1) |
92 (85:15) |
| 1b |
|
86 (44:56) |
92 (53:47) |
| 1c |
|
37 (>99:1) |
–d |
| 1d |
|
13 (1: >99)e |
29 (1: >99) |
| 1e |
|
60 (>99:1) |
91 (>99:1) |
| 1f |
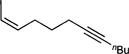
|
69 (>99:1) |
86 (>99:1) |
| 1g |
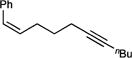
|
50 (>99:1) |
–d |
| 1h |
|
54 (49:51) |
–d |
| 1i |
|
57 (5:95)f |
–d |
| 1j |
|
36 (1:>99)g |
–d |
| 1k |
|
37 (1:>99)h |
–d |
| 11 |
|
0 | n.d. |
| 1m |
|
0 | n.d. |
Loading of [py2TiCl2NPh]2 and reaction yields with respect to PhNNPh.
Isolated as the ketone product after hydrolysis. See SI for details.
Determined by 1H NMR analysis of the crude reaction mixture.
Could not be determined due to peak overlap in the 1H NMR spectrum.
Isolated as the retro-ene product 3d′ (Figure 3).
As a mixture of 2i, 3i and 4i (Figure 5).
As 4j.
As a mixture of 3k and 4k.
