Table 2.
Intermolecular Multicomponent Carboamination of Alkynes and Alkenes with PhNNPha
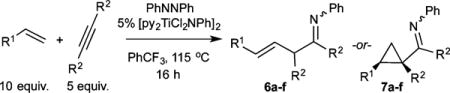
| ||||
|---|---|---|---|---|
|
| ||||
| Alkene | R2 | % Isolated Yield (6:7)b | 1H NMR % Yield (6:7)c | |
| 5a |
|
Me | 54 (40:60) | n.d. |
| 5b |
|
Et | 61 (>99:1) | n.d. |
| 5c |
|
Me | 42 (9:91) | 63 (15:85)d |
| 5d |
|
Et | 51 (>99:1) | 70 (71:29)e |
| 5ef |
|
Et | 40 (>99:1) | n.d. |
| 5f |
|
Et | 0 | n.d. |
Loading of [py2TiCl2NPh]2 and reaction yields with respect to PhNNPh.
Isolated as the ketone product after hydrolysis. See SI for details.
Determined by 1H NMR analysis of the crude reaction mixture.
96:4 ratio of cis:trans cyclopropane product.
74:26 ratio of cis:trans cyclopropane product.
Reaction run in neat alkene.
