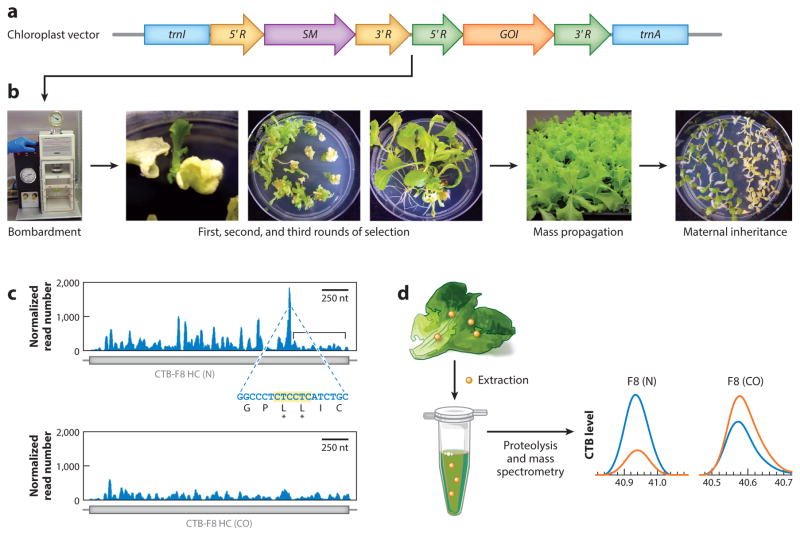
Figure 1.
Concepts of chloroplast transformation and characterization of transplastomic plants. (a) Representation of a chloroplast vector containing the most commonly used flanking sequences for homologous recombination, trnI and trnA (blue). The selectable marker (SM) gene ( purple) and gene of interest (GOI; orange) are regulated by promoters and 5′ untranslated regions (UTRs) (5′ R; yellow and green) to enhance transcription and translation and by 3′ UTRs (3′R; yellow and green) to enhance transcript stability. (b) The vector is bombarded into leaves with a gene gun. Homoplasmy is achieved after three rounds of selection on antibiotic-containing media, followed by mass propagation and verification of maternal inheritance. (c) Ribosome profiling demonstrates that ribosome stalling (blue peaks) occurs throughout a native human gene, Clotting Factor VIII (F8) (N; top), and especially in a region with consecutive lysines, but is substantially reduced when codons are optimized for plastid expression (CO; bottom). The bracketed region in the top graph indicates decreased ribosome occupancy of the transcript after the stalling site; this region is absent from the codon-optimized transcript, indicating increased translation of the entire open reading frame. (d ) Quantification of a therapeutic protein expressed in chloroplasts. Protein is extracted from transplastomic plants ( yellow dots), and the extract is subjected to proteolysis and mass spectrometry by parallel reaction monitoring. Levels of cholera toxin B (CTB) from the fusion protein (orange curves) are compared with standards (blue curves) and show higher levels in plants expressing codon-optimized human FVIII than in plants expressing the native gene. Panels c and d adapted with permission from Reference 70.