Abstract
Disruption of murine Hook1 results in a disturbed spermatogenesis and consequently leads to male infertility in mice. Within these mice abnormal sperm development starts with a disorganization of the microtubular manchette in elongating spermatids that leads to an abnormal head shape as well as to distinctive structural changes in the flagella of the sperm. To elucidate Hook1 function in male germ cell differentiation a yeast two hybrid screen was performed using a murine testicular library, which lead to the identification of several putative Hook1 interacting proteins. One of the isolated cDNA fragments encodes for the coiled-coil domain containing protein 181 (Ccdc181).
The putative interaction of Ccdc181 with Hook1 was verified by FRET analysis and interacting regions were identified using yeast two hybrid assays. Furthermore, Ccdc181 seems to interact directly with microtubules and localizes to the microtublar manchette of elongating spermatids, resembling the previously reported localization of Hook1. According to the observed immunostaining pattern the RNA expression of Ccdc181 is less prominent in pre-meiotic stages of sperm development but increases in the haploid phase of spermatogenesis and seems to be restricted to male germ cells. However, Ccdc181 expression is also observed to a lower extent in somatic tissues, particularly, in tissues containing ciliated epithelia. Additionally, Ccdc181 protein is found to localize to the sperm flagella and to the basal half of motile cilia, whereas Ccdc181 was not detected in primary non-motile cilia. Furthermore, we showed that Ccdc181 is a putative interacting partner of the different catalytic subunits of Pp1, raising the hypothesis that Ccdc181 plays a role in mediating ciliary motility.
Keywords: Ccdc181, Cilia, Manchette, Microtubules, Motility, Hook1, Spermatogenesis
Introduction
Spermatogenesis is characterized by successive periods of regulated cell proliferation, meiotic divisions and complex phases of differentiation of the newly formed haploid spermatids during spermiogenesis. Differentiation is characterized by the shaping and condensation of the nucleus as well as the formation of the acrosome and the development of the sperm tail. The formation of the acrosome-acroplaxome-manchette complex is essential for proper spermatid development (Kierszenbaum and Tres, 2004). While the acroplaxome anchors the developing acrosome to the elongating spermatid head and provides a mechanical scaffold for the shaping of the spermatid nucleus (Kierszenbaum and Tres, 2004), the manchette is a transient microtubular/actin-containing structure that occurs in elongating spermatids and disappears before spermatozoa are released to the epididymis. As motor proteins that might be involved in intraflagellar transport have also been localized to the manchette (Hayasaka et al., 2008), this structure is thought to serve in the transport of vesicles and macromolecules to the centrosomes as well as to the growing spermatid tail, a process comparable to the intraflagellar transport found in eukaryotic flagella and motile cilia (Kierszenbaum, 2002). Disruption of the manchette structure results in a disturbed transport along the microtubules of the manchette and ultimately leads to male infertility. In spermatids of azh-mutant mice the microtubules of the manchette are often ectopically positioned, leading to abnormal sperm head shape and malformed sperm tails. This phenotype is caused by a deletion of two exons of the Hook1 gene, suggesting that Hook1 seems to be essential for the proper function of the manchette during sperm development (Meistrich et al., 1990; Mendoza-Lujambio et al., 2002; Mochida et al., 1999). It has been shown that Hook1 can bind to the microtubules of the manchette and therefore it seems to play an essential role for the formation and maintenance of this organelle (Mendoza-Lujambio et al., 2002). In mammals Hook1 belongs to a family of Hook proteins, consisting of Hook1, Hook2 and Hook3. Hook2 has been shown to be involved in primary cilia morphogenesis (Baron Gaillard et al., 2011) while Hook3 has been shown to participate in the localization of the Golgi complex (Walenta et al., 2001). Hook polypeptides consist of a highly conserved NH2-domain mediating attachment to microtubules, a central coiled-coil domain capable of homodimerisation and a more divergent COOH-terminal organelle binding domain putatively involved in binding of specific organelles or cargos (Mendoza-Lujambio et al., 2002; Walenta et al., 2001). In Drosophila hook is predominantly expressed in testis but is also detected in somatic cells, where it was found to play a role in endocytosis of transmembrane ligands (Kramer and Phistry, 1999) and human HOOK1 functions as a microtubule-dependent cargo transporter for endosomal sorting (Maldonado-Baez et al., 2013). Since Hook1 was not detected in protein extracts of mature mouse sperms it is unlikely that Hook1 acts as a structural component of the spermatozoa, rather Hook1 might be involved in intra-manchette transport processes, sorting new synthesized polypeptides in the elongating spermatid to the correct compartment (Mendoza-Lujambio et al., 2002). To identify putative Hook1 cargoes in male germ cell development a yeast two hybrid screen using a testicular library was performed. By this approach the mouse coiled-coil domain containing protein 181 (Ccdc181) formerly known as 4930455F23 riken protein was isolated. Ccdc181 belongs to a group of polypeptides whose common pattern is the coiled-coil domain. However, less is known concerning the molecular function of Ccdc181 with the exception of it being predicted as a cilia-related gene and human CCDC181 being reported as a putative prognostic factor for prostate cancer (Haldrup et al., 2013; McClintock et al., 2008). Moreover, the murine Ccdc181 was identified as a putative interacting partner of CCNB1IP1, a RING domain-containing protein that is required for meiotic crossing over processes, and of protein phosphatase 1 subunit α (Pp1α), a ubiquitous expressed serine/threonine phosphatase involved in various cellular pathways (Doerks et al., 2002; Strong and Schimenti, 2010). The highly similar Pp1γ2 subunit has been shown to play an important role in sperm motility since the knockout of Pp1γ2 resulted in male infertility due to non-motile sperms. Additionally, it was shown that in testis Pp1γ2 function could not be substituted by other Pp1 catalytic subunits like in contrast to somatic tissues (Chakrabarti et al., 2007; Fardilha et al., 2011; Sinha et al., 2013). In the present study we report the molecular characterization of murine Ccdc181 within male germ cell development. We demonstrate that Ccdc181 is able to bind to Hook1 supporting the idea that Ccdc181 is a cargo of Hook1. Moreover, our results indicate that Ccdc181 can interact with Pp1γ2 and binds to microtubules of motile cilia and sperm flagella suggesting that Ccdc181 might play a role in the generation of motility in these organelles.
Materials and methods
Cell culture
Human HEK 293T and mouse NIH 3T3 cells were cultured in DMEM (GIBCO) supplemented with glutamine, 4.5 mg/ml glucose, 10% fetal bovine serum (FBS) and 100 μg/ml penicillin/streptomycin at 37°C and 5% CO2. HEK 293T or NIH 3T3 cells were transfected with the jetPRIME transfection reagent (Peqlab) according to the manufacturer’s protocol. Briefly, HEK 293T cells grown on 10 cm plates were transfected using 10 μg DNA and 30 μl transfection reagent, whereas NIH 3T3 cells grown on 6 cm plates were transfected using 6 μg DNA and 18 μl transfection reagent. For the induction of cilia formation in NIH 3T3 cells confluent cultures were serum starved for 24 hours prior further analysis (He et al., 2014).
Preparation of expression constructs
Expression constructs were prepared using primers with unique restriction sites. Hook1 full-length (NM_030014, aa 1-728) and Hook1-AZH (aa 1-263), as well as the truncations Hook1-D1 (aa 1-276), Hook1-D2 (aa 167-458) and Hook1-D3 (aa 459-728) and the deletions Hook1-ΔCC1 (aa 1-728, Δ173-228), Hook1-ΔCC2 (aa 1-728, Δ254-443) and Hook1-ΔSSF2 (aa 1-728, Δ185-283) were generated. Ccdc181 full-length (NM_029115, aa 1-509) as well as the truncations Ccdc181-D1 (aa 1-254), Ccdc181-D2 (aa 248-448) and Ccdc181-D3 (aa 393-509) were generated. For FACS-FRET Hook1 and Ccdc181 were cloned into pEGFP-N1 and DsRed-C1 (Clontech). Additionally, a fusion construct of GFP and DsRed was generated and a fusion construct of Tubulin and GFP was kindly provided by J. Schmid. For yeast-two-hybrid indicated Hook1 and Ccdc181 constructs as well as Hook2 (NM_133255, aa 1-716), Hook3 (NM_207659, aa 1-718) and catalytic subunits of Pp1 (Pp1α (NM_031868, aa 1-330), Pp1β (NM_172707, aa 1-327), Pp1γ1 (NM_013636, aa 1-323) and Pp1γ2 (aa 1-337)) were cloned into pGBKT7-BD (Clontech) and indicated Ccdc181 constructs were also cloned into pGADT7-AD (Clontech). For microtubule-binding assay, indicated Ccdc181 constructs were cloned into pEXPR-IBA43 (IBA).
FACS-FRET analysis
Transfected NIH 3T3 cells were harvested 24 hours post transfection by trypsinization (0.5 ml 0.05% Trypsin-EDTA, 5 minutes), resuspended in PBS and subjected to FACS-FRET analysis within one hour after preparation. FACS-FRET measurements were performed using a LSR Fortessa flow cytometer (BD Bioscience) or a CytoFlex S (Beckman Coulter) equipped with 405 nm, 488 nm, 561 nm and 640 nm lasers as described previously (Banning et al., 2010). The protocol was modified in regards of the set of fluorophores used. To measure GFP and FRET signals, cells were excited with the 488 nm laser and the fluorescence was collected using a standard 525/50 filter, while the FRET signal was measured with a 610/20 filter. To measure DsRed signals, cells were excited with the 561 nm laser while emission was taken with a 575/26 or 610/20 filter. To set up the gates cells expressing either GFP or DsRed, were used to exclude single transfected cells. Additionally, cells co-expressing GFP and DsRed, as well as cell expressing GFP-DsRed fusion protein were used to set up a gate to exclude FRET negative cells (Fig. S1). For each sample a minimum of 8,000 cells that fell within the background adjusted gate were evaluated.
Yeast-2-hybrid-assay
Yeast two-hybrid assays were performed according to the manufacturer’s protocol (Matchmaker Gold Yeast Two-Hybrid Manual, Clontech). Constructs were transformed into the AH109 yeast strain by the lithium acetate method (Gietz, 2014). Co-expression of transformed plasmids was tested on double drop-out plates lacking the amino acids leucine and tryptophan (DDO, SD/-Leu/-Trp). Interaction between two polypeptides was investigated on quadruple drop-out plates lacking the amino acids adenine, histidine, leucine and tryptophan (QDO, SD/-Ade/-His/-Leu/-Trp). Plates were incubated at 30°C for up to seven days until colonies became visible.
Reverse transcription PCR and quantitative RT-PCR analysis
Total RNA was extracted from mouse tissues using QIAshredder (Qiagen) and the Microarray Tissues Mini Kit (Qiagen). For quantitative expression analysis approximately 750 ng of extracted RNA were reverse transcribed using the QuantiTect Reverse Transcription Kit (Qiagen), according to the manufacturer’s protocol. Quantitative RT-PCR was performed using the CFX Connect Real-Time PCR Detection System (Bio-Rad) with the GoTaq qPCR Master Mix (Promega) according to the manufacturer’s protocol. Reactions were performed in triplicates with a single real-time PCR thermal profile (95°C for 10 min; 40 cycles of 95°C for 15 sec and 60°C for 30 sec). For the amplification the following primer combinations were used: qrtCcdc181-fwd 5’-CAAGAGGGAGGAAGAACAGC-3’ and qrtCcdc181-rev 5’-CGCATTTCTATGACCTGCTC-3’; qrtB2m-fwd 5’-GTCAGCATGGCTCGCTCGGT-3’ and qrtB2m-rev 5’-AGGCGGGTGGAACTGTGTTACG-3’; and qrtGadph-fwd 5’-CATCACCATCTTCCAGGAGC-3’ and qrtGadph-rev 5’-ATGACCTTGCCCACAGCCTT-3’. Expression levels of Ccdc181 were normalized to the expression of B2m and Gapdh and the relative quantification of gene expression was analyzed by the 2-ΔΔC(t) method (Livak and Schmittgen, 2001). For non-quantitative expression analysis extracted RNA was reverse transcribed using the AMV First Strand cDNA Synthesis Kit (New England Biolabs) and amplified using the DreamTaq Green PCR 2x Master Mix (Fisher Scientific) according to the manufacturer’s protocols. For the amplification the following primer combinations were used in the same reaction: rtCcdc181-fwd 5’-CTCAAGAGGGAGGAAGAACAG-3’ and rtCcdc181-rev 5’-CCTTCTGAGCCATTGTCG-3’; and rtGapdh-fwd 5’-AGGCCGGTGCTGAGTATGTC-3’ and rtGapdh-rev 5’-TGCCTGCTTCACCACCTTCT-3’.
Northern blotting
Total RNA was extracted from different mouse tissues and testes of different mouse mutants using the RNeasy Plus Mini Kit (Qiagen). Approximately 6 μg RNA were separated on an agarose gel under denaturing conditions. Separated samples were transferred onto a Hybond-N+ membrane (Amersham) using the TurboBlotter (Whatman) and cross-linked at 80 °C for 120 minutes. Hybridization and signal detection was performed using DIG DNA Labeling and Detection Kit (Roche) according to the manufacturer’s instructions. Briefly, hybridization was performed overnight at 50 °C in DIG Easy Hyb buffer (Roche), mixed with denatured dig-labelled Ccdc181 cDNA probe. Hybridized probes were labeled with alkaline phosphatase-coupled digoxigenine antibody (1:10,000) and detected using CDP Star Reagent (New England Biolabs) according to manufacturer’s protocol. The Ccdc181 cDNA probe was amplified with the following primers: nbCcdc181-for (5’-GATGAAGATAAAGATATTGATTCAAAAGAGAG-3’) and nbCcdc181-rev (5’-GTTGTAGTGGTCGGTGAAGC-3’) and dig-labelled using the PCR DIG Probe Synthesis Kit (Roche) according to manufacturer’s protocol.
Preparation of testis lysates
Testes of 5- to 50-day-old mice were homogenized with 1:20 (w/v) T-PER (Fisher Scientific) supplemented with 1x HALT protease inhibitor cocktail (Fisher Scientific) using the “TissueRuptor” (Qiagen) and cleared by centrifugation (20,000 g, 10 minutes, 4°C). Samples were analyzed by SDS-PAGE and Western blotting.
SDS-PAGE and Western blotting
Samples were denatured at 95°C for 5 minutes, separated by SDS-PAGE and transferred onto nitrocellulose membranes using the iBlot dry blotting system (Invitrogen). For immunodetection, rabbit polyclonal anti-Ccdc181 (1:1,000, Sigma-Aldrich) and anti-Gapdh (1:10,000, Cell Signaling), and mouse monoclonal anti-StrepTagII (1:10,000, IBA) and anti-α-Tubulin (1:1,000, Sigma-Aldrich) were used. Antibodies were detected using HRP-linked polyclonal goat anti-rabbit and anti-mouse antibodies (1:10,000 Bethyl). Signals were detected using Clarity Western ECL Substrate (Bio-Rad) and captured with the FluorChem HD2 imager (Protein Simple).
Preparation of testicular germ cells and tracheal epithelial cells
Mouse testicular germ cells were prepared as previously described (Bellve et al., 1977; Mendoza-Lujambio et al., 2002) with minor modifications. In brief, testes harvested from a C57BL/6 mouse were decapsulated, immersed in M2 medium (Sigma-Aldrich) supplemented with 0.5 mg/ml collagenase (Sigma-Aldrich) and incubated for 15 minutes at 33°C. The dispersed seminiferous tubules were allowed to sediment for 3 minutes and were washed with M2 media for three times. After that the tubules were taken up in M2 media supplemented with 0.5 mg/ml trypsin EDTA and 1 μg/ml DNase-I (New England Biolabs) and incubated for 15 minutes at 33 °C. Remaining cell aggregates were sheared by gentle pipetting. Cells were washed two times in M2 media, centrifuged (200 g, 5 min), resuspended in M2 media containing 0.5 % BSA and filtered through a 40 μm nylon mesh. Finally, two drops of the solution were placed on a slide and allowed to dry at room temperature.
The trachea of an adult C57BL/6 mouse was isolated and single tracheal cartilages were cut using a fine scissor, placed in drops of PBS on slides, dissected with fine forceps and allowed to dry at room temperature.
Immunostaining of tissue sections
Mouse testis, lung, trachea and brain were fixed using Bouin’s solution (Sigma-Aldrich), dehydrated and embedded in Parablast-Xtra (Sigma-Aldrich). Sections of theses tissues as well as of commercially available mouse reproductive tissues (MAP-400, Amsbio) were deparaffinated using Roticlear (Carl Roth) and hydrated by descending alcohol concentrations. Antigens were unmasked by incubation of slides in modified citrate buffer, pH 6.1 (Dako) for 10 min at 120°C. After that, sections were permeabilized for 10 min with 0.2% Triton X-100 in PBS. For chemiluminescence staining slides were incubated with BloxAll solution (Vectorlabs) for 10 min, blocked with IB-solution (10% Roti-Immunoblock, 5% normal goat serum and 3% BSA in PBS) for two hours and incubated with rabbit polyclonal anti-Ccdc181 (1:100, Sigma-Aldrich) overnight at 4°C. The next day, slides were incubated with polyclonal goat anti-rabbit AP-conjugated antibody (1:50, Sigma-Aldrich) for 90 minutes and stained using the Vector Red AP Substrate Kit (Vectorlabs) according to the manufacturer’s protocol, counterstained with Mayer’s Hematoxylin (Sigma-Aldrich), mounted with RotiMount (Carl Roth) and examined using the Zeiss TissueFAXs. For immunofluorescence staining slides were incubated with ImageIT-FX (Invitrogen) for 30min and with M.O.M. (Vectorlabs) for one hour. After blocking with IB-Solution for two hours, slides were incubated with rabbit polyclonal anti-Ccdc181 (1:100, Sigma-Aldrich) and mouse monoclonal anti-acetylated α-Tubulin (1:2,000, Sigma-Aldrich) antibodies overnight. The next day, slides were incubated with goat anti-mouse Alexa488- and goat anti-rabbit Alexa568-coupled antibodies (1:500, Invitrogen) for 90 minutes, mounted with Prolong Diamond Antifade with DAPI (Invitrogen) and examined using the Zeiss LSM780 laser scanning microscope.
Immunofluorescence staining of cells
Cells were fixed with 4% paraformaldehyde in PBS (Sigma-Aldrich) for 15 minutes and permeabilized with 0.5% Triton X-100 in PBS for 15 minutes. Slides were blocked with IB-solution for two hours and incubated either with rabbit polyclonal anti-Ccdc181 (1:100, Sigma-Aldrich) and mouse monoclonal anti-α-Tubulin (1:500, Sigma-Aldrich) or mouse monoclonal anti-acetylated α-Tubulin (1:2,000, Sigma-Aldrich), or with rabbit polyclonal anti-Ccdc181 (1:100, Bioss Antibodies) and anti-Hook1 (1:100, Bios Antibodies) directly coupled to Alexa488 or Alexa555, respectively, over night at 4°C. The next day, slides incubated with unlabeled primary antibodies were incubated with goat anti-mouse Alexa488- and goat anti-rabbit Alexa568-coupled antibodies (1:500, Invitrogen) for 90 minutes. Additionally, to visualize the acrosome of isolated germ cells all slides were incubated with Alexa647-conjugated PNA (1:100, Invitrogen) in PBS for 30 minutes. All slides were mounted using ProLong Diamond Antifade with DAPI (Invitrogen) and examined using the Zeiss LSM780 laser scanning microscope.
Microtubule binding assay
Transfected HEK 293T cells were harvested 48 hours post transfection with ice-cold microtubule binding buffer (80 mM Pipes, 5 mM MgCl2, 0.5 mM EGTA, 5% glycerol) supplemented with 1x HALT Protease Inhibitor Cocktail (THP Medical Products) and lysed using the Precellys-24 (PeqLab Biotechnology) at 4,500 rpm for 1 minute. Protein lysates were cleared by ultracentrifugation (100,000 g, 40 minutes) and stored on ice until further use. In vitro microtubule binding assay was performed using the microtubule binding protein spin down assay kit (Cytoskeleton) according to the manufacturer’s protocol. Briefly, 20 μl in vitro polymerized microtubules were mixed with 30 μl cleared protein lysate and incubated for 30 minutes at room temperature. As a control, lysates were incubated in the absence of polymerized microtubules. Microtubules and binding proteins were sedimented through 100μl of a 40% glycerol cushion by ultracentrifugation (100,000 g, 40 minutes). Pellet and supernatant samples were analyzed by SDS-PAGE and Western blotting.
Statistical analysis
GraphPad Prism was used for statistical analysis and graph preparation. Error bars indicate standard error of the mean (SEM). Differences were considered significant when P was 0.05 or less in an unpaired student t-test. Significant differences were ***P <0.001, ****P <0.0001.
Results
Identification of murine Ccdc181 cDNA using the yeast two hybrid system
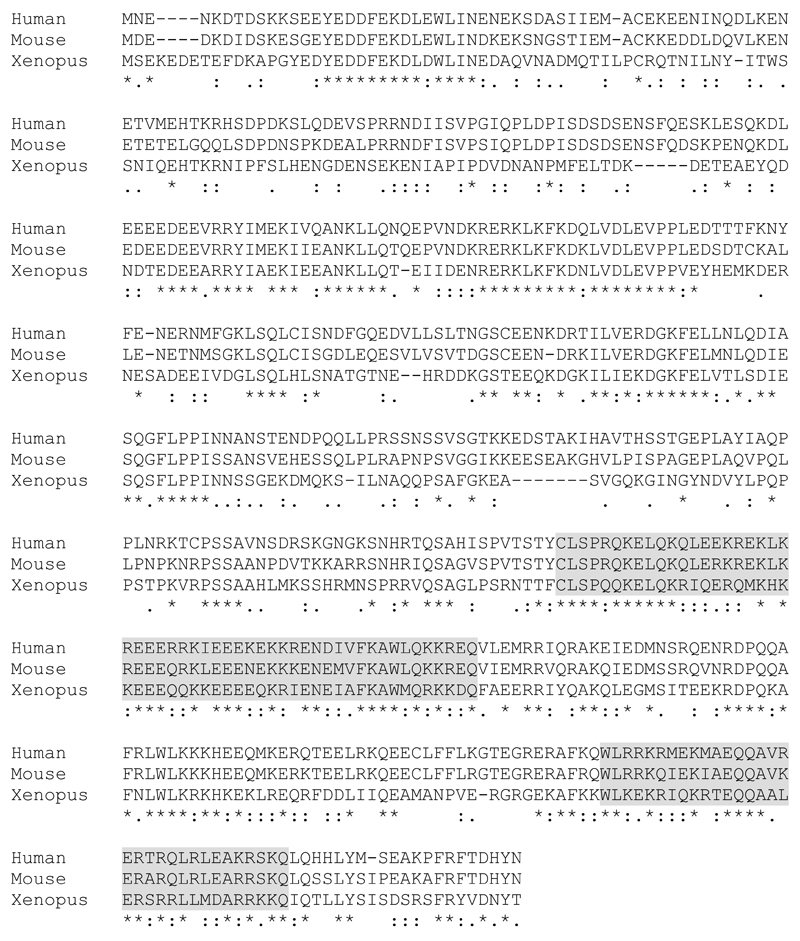
To identify putative Hook1 interacting proteins a yeast two hybrid screen using a murine testicular library was performed and resulted in the isolation of more than twenty different putative Hook1 interaction partners. One of the identified clones contained a 417 base pair fragment corresponding to the Mus musculus RIKEN cDNA 4930455F23 gene (GenBank BC048086.1; NCBI Reference Sequence: NM_029115.3), which was later renamed to Ccdc181. The Ccdc181 gene is located at mouse chromosome 1, consists of six exons and encodes a 1,909 base pair large transcript with 509 amino acid residues and a predicted molecular weight of 59.25 kDa. Ccdc181 belongs to the coiled-coil domain containing protein family, which includes various so far uncharacterized proteins, containing at least one coiled-coil domain. However, only low similarities between Ccdc181 and other members of this family were found by multi-alignment analysis (data not shown). To assess the level of conservation of Ccdc181, we compared protein sequences of the Ccdc181 homologs in human, mouse and xenopus using Clustal Omega (Goujon et al., 2010; Sievers et al., 2011). This revealed evolutionary highly conserved regions at the N- and C-terminus of the Ccdc181 protein sequence (Fig. 1). Moreover, bioinformatics analysis of mouse Ccdc181 using the ExPASy coils server (Lupas et al., 1991) identified two putative coiled-coil domains at the C-terminal part of Ccdc181 at amino acid residues 333 to 385 and 458 to 488 (Fig. 1, grey boxes).
Figure 1. Ccdc181 contains evolutionary conserved regions.
Protein alignment of human (NP_001287898, Q5TID7), mouse (NP_083391, Q80ZU5) and xenopus (F6UGJ0) Ccdc181 orthologues using Clustal Omega. Symbols for identical (*), conserved (:) and semi-conserved (.) amino acids are displayed below the alignments. Gray highlighted amino acids indicate putative coiled-coil motifs as predicted by the ExPASy coils server.
Ccdc181 interacts with Hook1
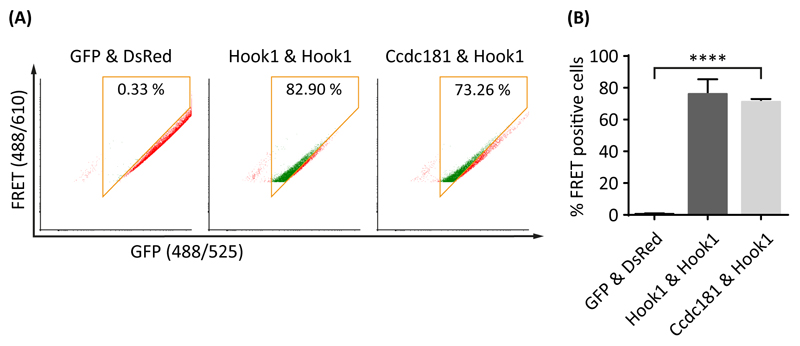
To confirm the putative interaction of Ccdc181 and Hook1 found by yeast two hybrid, we performed a FACS (fluorescence-activated cell sorting) based FRET (fluorescence resonance energy transfer) analysis. For this, gates were set up to exclude single transfected and FRET negative cells. Therefore, cells expressing either GFP-only or DsRed-only were analyzed together with cells co-expressing GFP and DsRed and cells expressing a GFP-DsRed fusion protein (Fig. S1). For the analysis, cells co-expressing GFP-tagged Ccdc181 and DsRed-tagged Hook1 were tested for FRET positive cells. Because of Hook1’s known ability to form homodimers (Mendoza-Lujambio et al., 2002), cells co-expressing GFP-tagged and DsRed-tagged Hook1 were used as an internal positive control, whereas cells co-expressing GFP-only and DsRed-only were used as a negative control. In samples of cells co-expressing Ccdc181 and Hook1 71.17% (±1.05% SEM) of the cells were FRET positive, comparable to samples of the positive control with 76.12% (±5.36% SEM) FRET positive cells and significantly higher than samples of the negative control with 0.64% (±0.16% SEM) FRET positive cells, strongly supporting the proposed interaction of Ccdc181 and Hook1 (Fig. 2).
Figure 2. Ccdc181 is a Hook1 interacting protein.
Interaction between Ccdc181 and Hook1 was verified using a FACS based FRET analysis. (A) Representative FACS-plots showing the amount of FRET positive cells in living NIH 3T3 cells co-expressing the indicated GFP and DsRed fusion proteins. FRET positive cells within the gates (orange box) are displayed in green, while FRET negative cells outside the gates are displayed in red. Numbers give total percentages of cells within the FRET gate. The non-interacting proteins GFP and DsRed were used as a negative control, whereas Hook1’s ability to form homodimers was used as a positive control. (B) Quantification of FRET positive cells using mean values and standard error of the mean for the total amount of FRET positive cells from three independent experiments, that were analyzed as depicted in (A). P ≤ 0.0001.
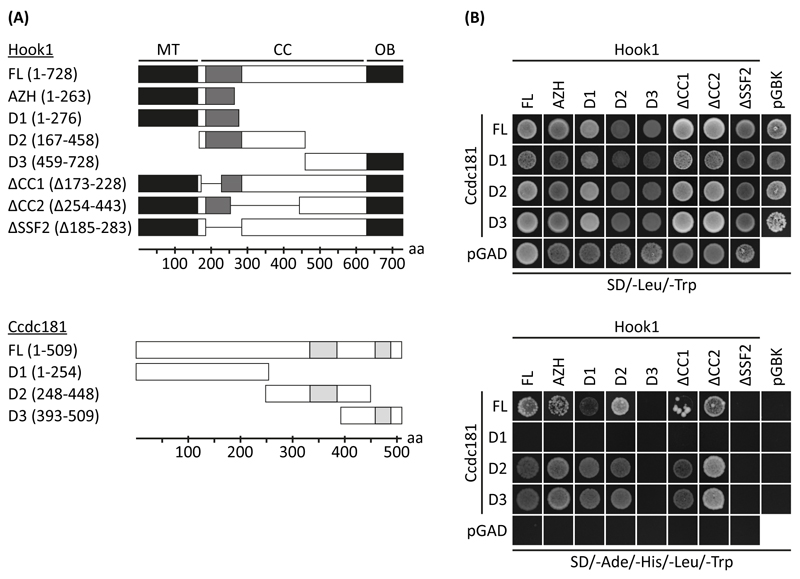
To further elucidate the interacting regions of Hook1 and Ccdc181, we prepared different truncation and deletion constructs for use in yeast two hybrid assays (Fig. 3A). The co-expression of these constructs was verified by yeast growth on double dropout plates (SD/-Leu/-Trp), whereas interacting constructs were identified by yeast growth on quadruple dropout plates (SD/-Ade/-His/-Leu/-Trp). As shown in Fig. 3B, the truncation mutants Hook1-D1 (amino acids 1-276) and Hook1-D2 (amino acids 167-458), as well as Hook1-AZH (amino acids 1-263) were able to interact with Ccdc181, whereas no interaction was observed for Hook1-D3. Since the interacting region seems to be restricted to the overlapping part of Hook1-D1 and Hook1-D2, we next used the two coiled-coil domain deletion constructs Hook1-ΔCC1 and Hook1-ΔCC2 lacking amino acids 173 to 228 and 254 to 443 respectively, as well as the SSF46579 domain deletion construct Hook1-ΔSSF2 lacking amino acids 185 to 283. Interaction of Ccdc181 and Hook1 was still observed in constructs lacking one of the coiled-coil domains (Hook1-ΔCC1, Hook1-ΔCC2) whereas the deletion of the SSF domain (Hook1-ΔSSF2) prevented interaction (Fig. 3B). Therefore, amino acids 229 to 253 of Hook1 seem to be most critical for its interaction with Ccdc181. For Ccdc181 the truncation mutants Ccdc181-D2 (amino acids 248-448) and Ccdc181-D3 (amino acids 393-509) were identified to interact with Hook1, whereas no interaction was observed for Ccdc181-D1 (amino acids 1-254) (Fig. 3B). Therefore, the amino acids 393 to 448 of Ccdc181, resembling the overlapping region of Ccdc181-D2 and Ccdc181-D3 seem to be most critical for its interaction with Hook1. Because Hook1 shares high similarity with Hook2 and Hook3, we next asked whether Ccdc181 is also able to interact with the two other Hook family members. Therefore, interaction of Ccdc181 with full length Hook2 and Hook3 were tested by a yeast two hybrid assay. As shown in Fig. S2A interaction of Ccdc181 with Hook2 and Hook3 was observed. Moreover, the interacting region of Ccdc181 was identified to be the same for all Hook family members. Using the yeast two hybrid approach we also tested the capability of Ccdc181 to form homodimers, by using combinations of the three different truncation constructs together with the full length Ccdc181 construct. The observed interaction between Ccdc181-D1 with either Ccdc181-D2 or Ccdc181-D3 suggests that Ccdc181 is at least in yeast able to form homodimers (Fig. S2B).
Figure 3. Identification of Ccdc181 and Hook1 interacting domains.
(A) Schemes of Hook1 and Ccdc181 truncation and deletion constructs showing putative coiled-coil domains (light grey) and the SSF46579 domain (dark grey). FL, full-length protein; MT, microtubule binding domain; CC, coiled-coil rich region; OB, putative organelle binding domain; aa, amino acids. (B) Yeast cells harboring indicated combinations of Ccdc181 constructs in pGADT7 or empty pGADT7 and Hook1 constructs in pGBKT7 or empty pGBKT7 were grown on medium lacking leucine and tryptophan (double dropout) to verify co-transformation. Interaction was assessed by analyzing yeast growth on medium lacking adenine, histidine, leucine and tryptophan (quadruple dropout). Deletion of SSF2 inhibits the interaction between Ccdc181, Ccdc181-D2 or Ccdc181-D3 with Hook1.
Expression analysis of the murine Ccdc181 gene
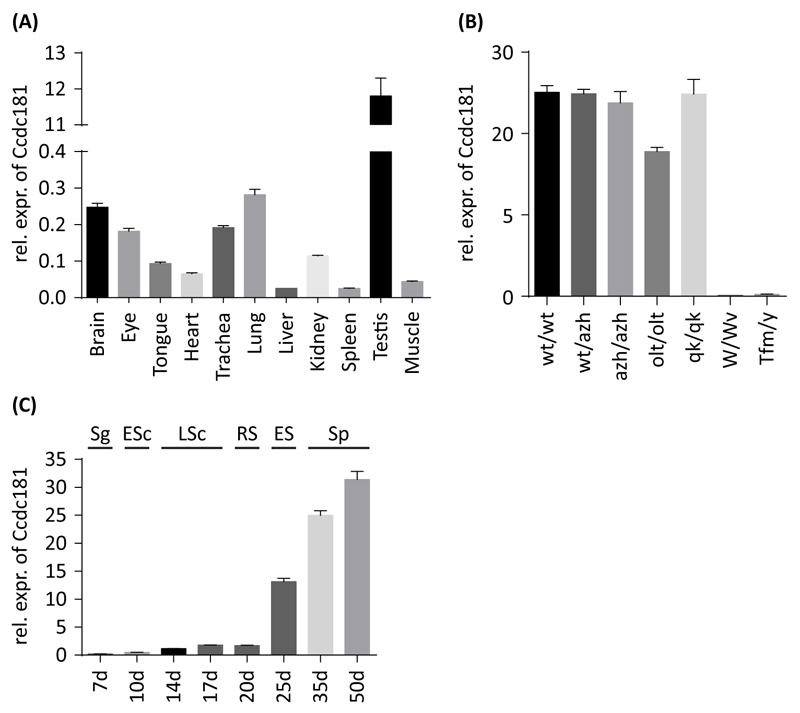
After the interaction between Hook1 and Ccdc181 was verified we next analyzed the expression pattern of Ccdc181 in testis and different somatic tissues. Therefore, RNA from different tissues of adult mice was extracted and analyzed using quantitative RT-PCR (Fig. 4A), RT-PCR (Fig. S3A) and Northern blot (Fig. S3B). The highest level of Ccdc181 expression was observed in testis, while in brain, eye, trachea and lung lower expression levels were detected. In tongue, heart, liver, kidney, spleen and muscle Ccdc181 transcripts were barely detectable (Fig. 4A). The difference in Ccdc181 expression levels between testis and somatic tissues were supported by RT-PCR and Northern blot results (Fig. S3A and B).
Figure 4. Expression analysis of Ccdc181 in mouse tissues, mutants and developmental male germ cell stages.
Ccdc181 expression was analyzed by quantitative RT-PCR and expression levels normalized to B2m and Gapdh are depicted as means ± SEM. (A) Expression of Ccdc181 in mouse tissues revealed elevated expression in testis. (B) In different mouse mutants, no Ccdc181 expression was observed in mutants lacking either germ cells (W/Wv) or haploid spermatids (Tfm/y). (C) Analysis of Ccdc181 expression during postnatal murine testicular development, revealed a sharp increase in Ccdc181 expression with the first occurrence of elongating spermatids. Sg, spermatogonia; ESc, early spermatocytes; LSc, late spermatocytes; RS, round spermatids; ES, elongating spermatids; Sp, mature spermatozoa.
To further investigate the expression of Ccdc181 in testis in more detail, testes of different mouse mutants were analyzed by quantitative RT-PCR (Fig. 4B). In azh/azh mutant mice, where Hook1-deletion leads to malformed sperms (Mendoza-Lujambio et al., 2002), as well as in quaking (qk/qk) and oligotriche (olt/olt) mutant mice, in which sperm tails are either abnormally developed or absent (Chubb, 1992), no difference in Ccdc181 expression was observed. However, in testicular feminization (Tfm/y) and Kit (W/Wv) mutant mice, where either spermatogenesis is arrested in first meiotic division (Lyon et al., 1975) or no germ cells are present within the testis (Ohta et al., 2003), no Ccdc181 expression was detected (Fig. 4B). These results were also supported by RT-PCR and Northern blot experiments (Fig. S3C and D). Therefore, Ccdc181 expression in mature mouse testis seems to be predominantly restricted to post meiotic germ cells. To further elucidate Ccdc181 expression during testis development, quantitative RT-PCR analysis of testes of 7-50 day-old mice was performed (Fig. 4C). Observed expression levels of Ccdc181 remained low until day 20, when round spermatids appear for the first time. After day 20 a sharp and constant increase in Ccdc181 expression was observed. A performed RT-PCR supported the observed elevated expression of Ccdc181 in later stages of spermatogenesis (Fig. S3E). This further confirms that Ccdc181 expression predominantly occurs in spermiogenesis.
Expression and localization analysis of the Ccdc181 protein
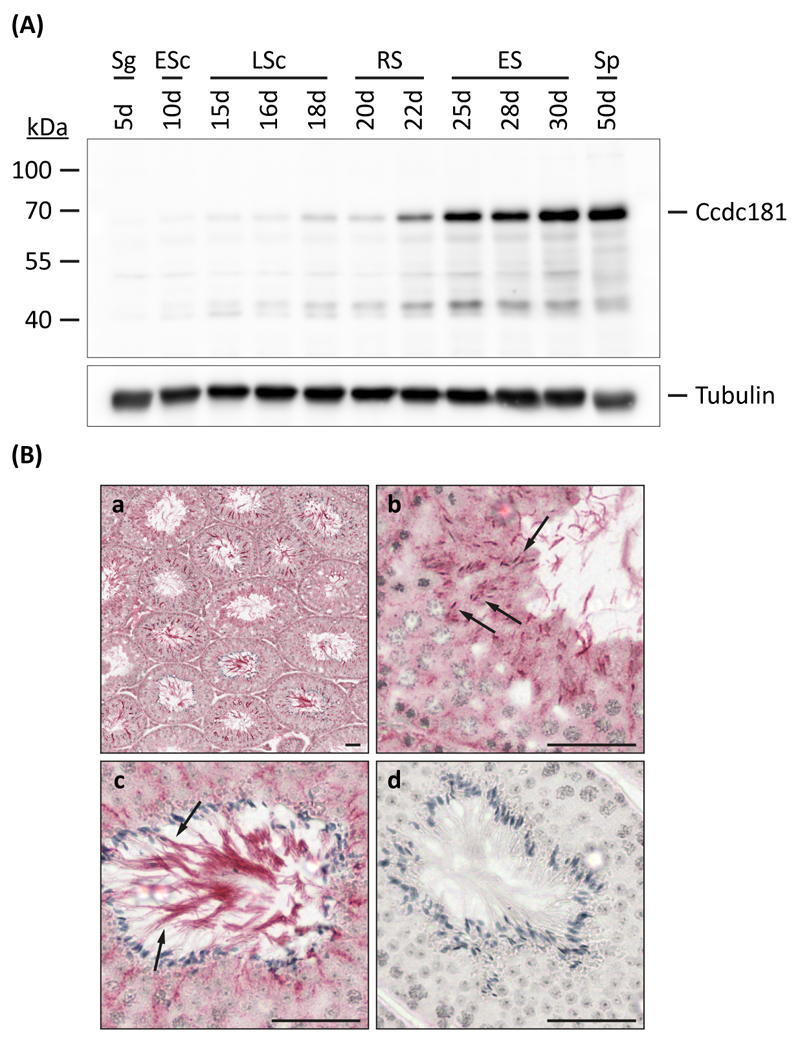
Since Ccdc181 RNA expression occurred mostly in spermiogenesis, we next wanted to investigate Ccdc181 protein expression during testis development. Therefore, testicular proteins were extracted from different aged male mice and probed with the Ccdc181 antibody, while the amount and integrity of protein extracts were analyzed using an α-Tubulin antibody. Similar to the RNA expression pattern of Ccdc181, only low Ccdc181 protein levels were observed until the first appearance of round spermatids. However, Ccdc181 protein levels sharply increase around day 25 with the onset of spermatid elongation (Fig. 5A). To further investigate the localization of Ccdc181 within differentiating germ cells, immunohistochemical experiments were performed. Therefore, paraffin sections of adult murine testes were probed with Ccdc181 antibody. Strong Ccdc181 expression was found in elongating spermatids, lying closely to the lumen of the tubules, whereas round spermatids showed weaker labeling. Further analysis revealed that Ccdc181 stains the microtubular manchette of these cells as well as the sperm tail (Fig. 5B).
Figure 5. Expression and localization of Ccdc181 protein.
(A) Protein expression of Ccdc181 during postnatal murine testicular development was analyzed by Western blot using testicular protein extracts of 5- to 50-day-old mice. Ccdc181 expression levels sharply increase around day 25 with the onset of spermatid elongation. Staining of α-Tubulin served as a loading control. Sg, spermatogonia; ESc, early spermatocytes; LSc, late spermatocytes; RS, round spermatids; ES, elongating spermatids; Sp, mature spermatozoa. (B) Localization of Ccdc181 was analyzed by immunohistochemical staining of adult mouse testis sections (a). Ccdc181 staining of the microtubular manchette in elongating spermatids (b, arrows) and the sperm tail (c, arrows) was observed. As a control staining without Ccdc181 antibody was performed (d). Scale bars: 50 μm.
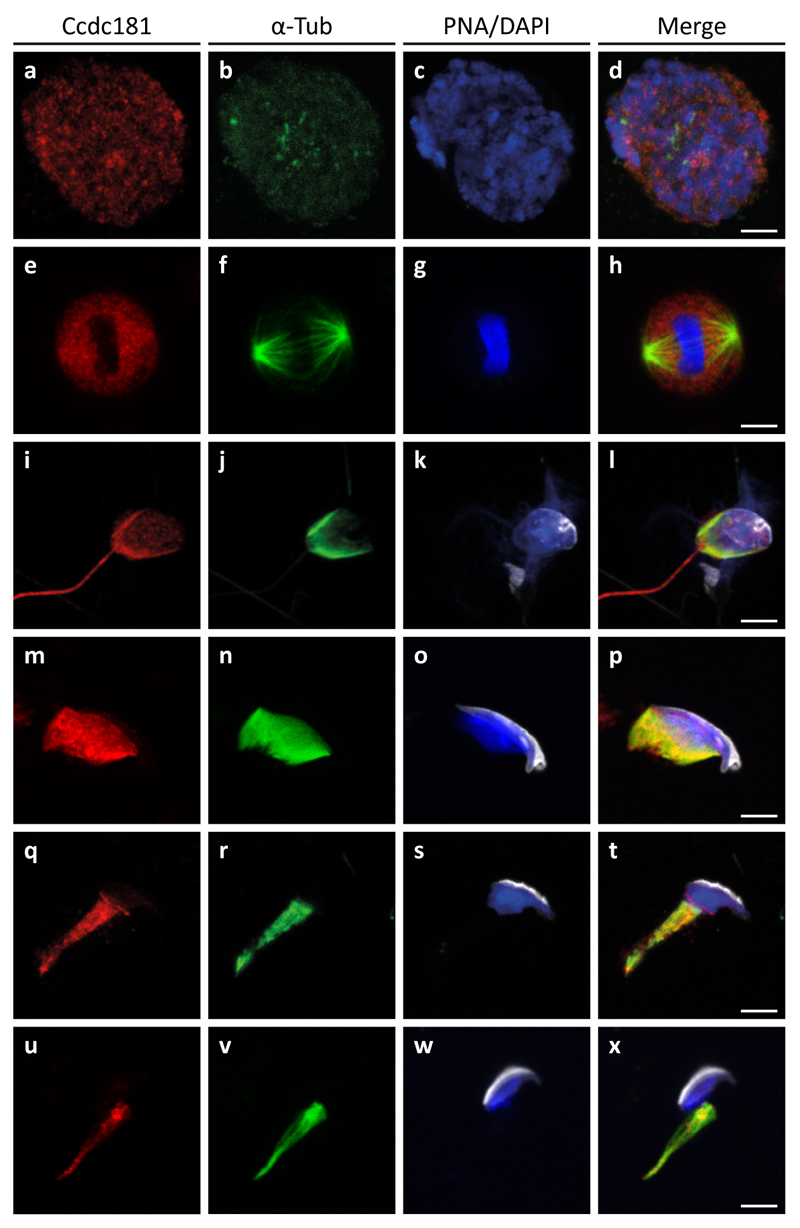
In order to determine the localization of Ccdc181 more precisely, germ cell suspension preparations were stained for Ccdc181 (red) and α-Tubulin (green). Additionally, the acrosome of developing spermatids and the nuclei were visualized using peanut-lectin (white) and DAPI (blue), respectively (Fig. 6). In early stages of male germ cell development only a diffuse staining of Ccdc181 in spermatocytes was detected (Fig. 6, a-d). During meiotic division a weak accumulation of Ccdc181 at the spindle apparatus was observed (Fig. 6, e-h). In more differentiated spermatids the manchettes and the elongating flagella are stained by α-Tubulin and the Ccdc181 antibodies, whereas in the remaining cytoplasm of the spermatids Ccdc181 is barely detectable (Fig. 6, i-p). During further spermatid development when the cytoplasm elongates Ccdc181 remains localized to the manchette (Fig. 6, q-x). As a similar localization pattern was previously described for Hook1 (Mendoza-Lujambio et al., 2002) and our in-vitro experiments have identified Hook1 and Ccdc181 as interacting proteins we next analyzed whether Ccdc181 and Hook1 co-localize in male germ cells. Therefore, germ cell preparations were analyzed for localization of Ccdc181 (green) and Hook1 (red) and revealed a co-localization of both polypeptides in spermatocytes and elongating haploid male germ cells (Fig. S4). Next the localization of Ccdc181 in the head region of an early elongating spermatid was investigated in more detail. Ccdc181 not only localizes to the microtubular manchette, but also decorates the nuclear ring and the most distal part of the microtubular manchette, whereas Ccdc181 signals showed no overlap with acrosome staining (Fig. 7A).
Figure 6. Distribution of Ccdc181 in developing male germ cells.
Testicular germ cells were prepared from adult C57BL/6 mice and indirect immunofluorescence staining was performed using polyclonal anti-Ccdc181 (red) and monoclonal anti-α-Tubulin (green) antibodies. Additionally, acrosomes were visualized by peanut-lectin (white) and nuclei were stained with DAPI (blue). (a-d) In premeiotic male germ cells only a diffuse staining of Ccdc181 was observed. (e-h) During meiotic division Ccdc181 faintly accumulates at the spindle apparatus. (i-x) In haploid male germ cell stages Ccdc181 staining is concentrated at microtubular manchettes and sperm flagella. Scale bars: 5 μm.
Figure 7. Ccdc181 localizes to distinct structures of the elongating spermatid.
(A) Indirect immunofluorescence staining of an elongating spermatid was performed using polyclonal anti-Ccdc181 (red) and monoclonal anti-α-Tubulin (green) antibodies. Additionally, acrosomes were visualized by peanut-lectin (white) (d-f) and nuclei were stained with DAPI (blue) (f). Ccdc181 staining overlaps with the microtubular manchette (a-c), however additional Ccdc181 signals were detected at the nuclear ring (c, arrow heads) and the distal end of the manchette (c, arrows). No overlap between Ccdc181 and Tubulin staining with the acrosome was observed (d-f). (B) The distribution of the relative fluorescence intensities of the different signals along the spermatid-head (A-f, along the arrow) support the Ccdc181 specific staining at the distal end of the microtubular manchette (black arrow) and the nuclear ring (black arrowhead). Scale bar: 5 μm.
Measurement of the relative fluorescence intensities along the elongating spermatid head supported the observed overlapping staining pattern of Tubulin and Ccdc181, whereas the most intense Ccdc181 staining was found at the distal tip of the manchette that elongates during development and surrounds the growing spermatid tail (Fig 7B). Taken together, these results suggest that Ccdc181 associates with microtubular structures, like the microtubular manchette and the sperm flagellum.
Localization analysis of Ccdc181 in cilia
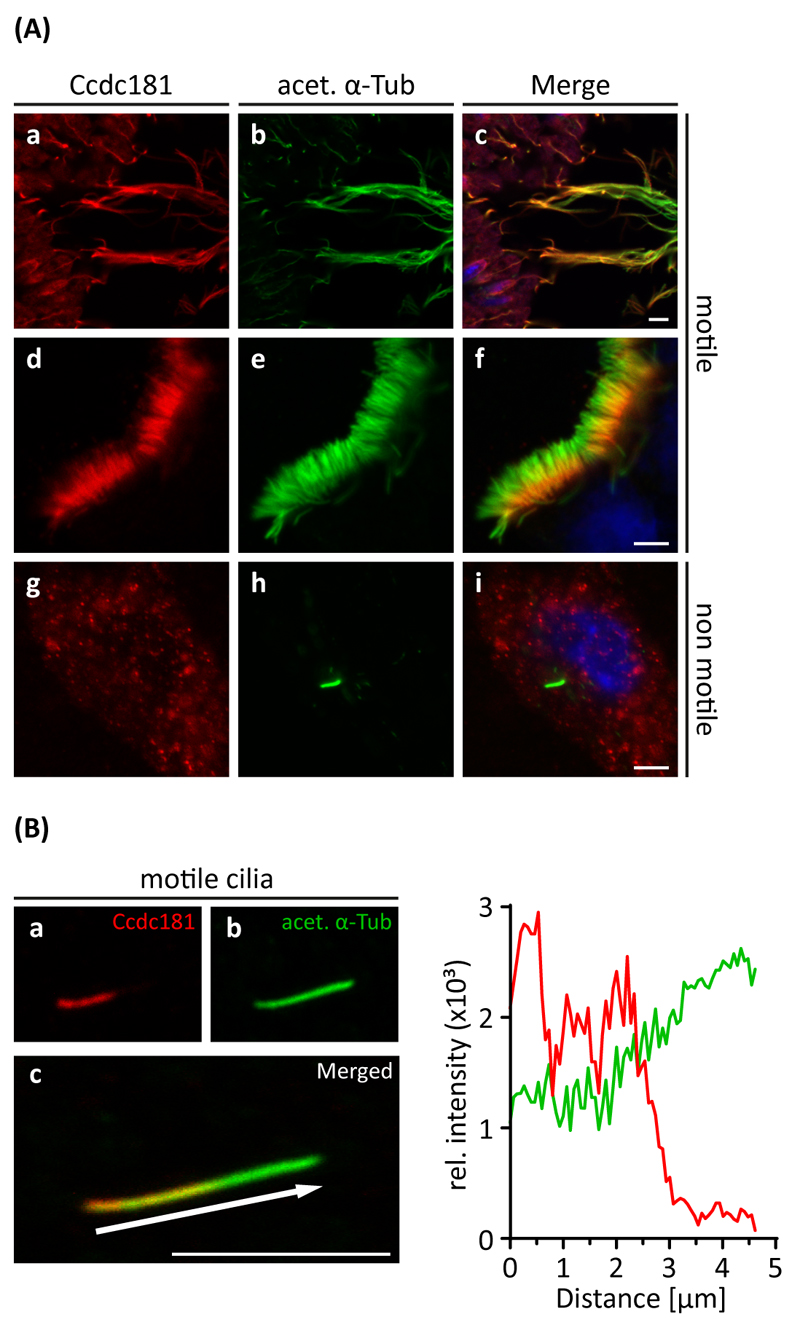
Because of the observed localization of Ccdc181 in sperm flagella, we next asked whether Ccdc181 also localizes to other ciliary structures. Therefore, paraffin sections of oviduct, brain, trachea and lung were analyzed for Ccdc181 localization. In these tissues Ccdc181 staining was exclusively observed in cilia of the epithelial layer (Fig. S5). In addition, testis flagella, cilia of the tracheal epithelial layer and primary cilia of NIH 3T3 cells were stained for Ccdc181 and acetylated α-Tubulin. Interestingly, Ccdc181 staining was observed in flagella and motile cilia, but not in non-motile primary cilia of NIH 3T3 cells. Moreover, Ccdc181 was observed to accumulate at the midpiece of spermatozoon tail and the basal half of tracheal cilia (Fig. 8A). Relative fluorescence intensity analysis of a single tracheal motile cilium confirmed our previous finding of Ccdc181’s localization at the proximal part of motile cilia, in contrast to acetylated α-Tubulin which stained the whole cilium. This indicates that Ccdc181 might be a component of the apparatus necessary for the generation of ciliary motility, rather than a component of the ciliary microtubule skeletal structure which is found to be similar in motile and non-motile cilia.
Figure 8. Ccdc181 localizes to motile cilia but not to non-motile primary cilia.
(A) Indirect immunofluorescence of Ccdc181 in sections of adult mouse testis (a-c), in dissected tracheal cartilages (d-f) and in NIH 3T3 cells induced for primary cilia formation (g-i) was performed using polyclonal anti-Ccdc181 (red) and monoclonal anti-acetylated α-Tubulin (green) antibodies. Additionally, nuclei were stained with DAPI (blue) (c, f, i). Ccdc181 predominantly localizes to sperm flagella, with a more intense staining at the midpiece (a-c). In tracheal epithelial cells, Ccdc181 was observed to localize to the basal part of motile cilia (d-f). In contrast, in NIH 3T3 cells Ccdc181 staining was absent in non-motile primary cilia, but was diffusely distributed in the cytosol (g-i). (B) The distribution of the relative fluorescence intensities of Ccdc181 and acetylated α-Tubulin along a single tracheal cilium (a-c, c arrow) is shown. In contrast to acetylated α-Tubulin, Ccdc181 predominantly localizes to the basal part of the tracheal cilium. Scale bar: 5 μm.
Ccdc181 is a microtubule-binding protein
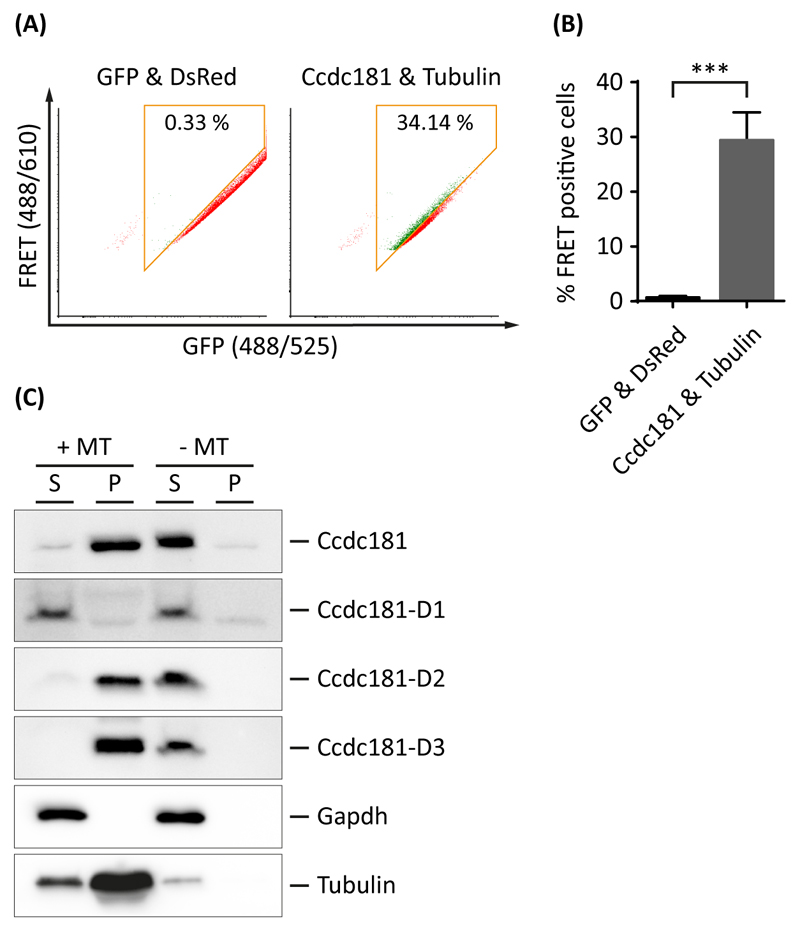
As previous results indicated a localization of Ccdc181 at microtubular structures such as the manchette or the axoneme of motile cilia, we were interested if Ccdc181 is also able to directly bind to microtubules. Therefore, a FACS based FRET analysis was performed with cells co-expressing DsRed-tagged Ccdc181 and GFP-tagged Tubulin. Control experiments with positive and negative FRET controls were performed as described before (Fig. S1). Approximately 29.42% (±2.92% SEM) of Ccdc181 and Tubulin co-expressing cells were found to be FRET-positive, indicating a direct interaction of Ccdc181 and Tubulin (Fig. 9A and B). Additionally, an in vitro microtubule binding protein spin-down assay was performed to further strengthen these findings and to investigate the responsible microtubule interacting domain of Ccdc181. Therefore, purified bovine brain tubulin was polymerized into microtubules and stabilized with taxol. Pre-cleared lysates of cells expressing StrepTag-conjugated Ccdc181 were either incubated with or without polymerized microtubules before sedimentation by ultracentrifugation was performed. In samples incubated with polymerized microtubules, Ccdc181 as well as Ccdc181-D2 and Ccdc181-D3 proteins were found in the pellet, whereas Ccdc181-D1 remained in the supernatant. In contrast, in samples lacking polymerized microtubules all Ccdc181 protein variants remained in the supernatant, indicating a specific interaction between Ccdc181 and microtubules. Gapdh and Tubulin staining were used to verify that only microtubules and microtubule-binding proteins were sedimented to the pellet (Fig. 9C). Taken together, our findings strongly suggest that Ccdc181 is a microtubule binding protein and that this interaction is mediated via the overlapping part of Ccdc181-D2 and Ccdc181-D3, previously identified as the necessary region for Hook1 interaction.
Figure 9. Ccdc181 is a microtubule-binding protein.
(A) Representative FACS-plots showing the amount of FRET positive cells in living NIH 3T3 cells co-expressing either GFP and DsRed or Ccdc181-GFP and Tubulin-DsRed proteins. FRET positive cells within the gates (orange box) are displayed in green, while FRET negative cells outside the gates are displayed in red. Numbers give total percentages of cells within the FRET gate. (B) Quantification of FRET positive cells using mean values and standard error of the mean for the total amount of FRET positive cells from three independent experiments, that were analyzed as depicted in (A). P ≤ 0.001. (C) A microtubule in vitro binding assay was performed using total protein lysates of HEK 293T cells overexpressing indicated Strep-tagged constructs which were incubated with (+MT) or without (-MT) stabilized microtubules. After sedimentation by ultracentrifugation proteins of the supernatant (S) and the pellet (P) were separated by SDS-PAGE. Distribution of different Ccdc181 proteins between supernatant and pellet fraction was analyzed using anti-Strep antibody. Gapdh and Tubulin distribution was used as negative and positive control, respectively. An interaction with microtubules was observed for Ccdc181, Ccdc181-D2 and Ccdc181-D3.
Ccdc181 is a putative interaction partner of catalytic subunits of Pp1
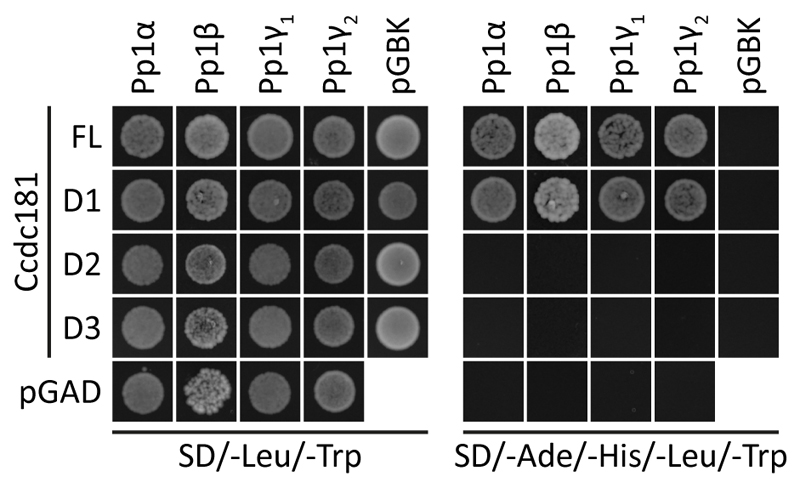
Previously, interaction of human CCDC181 with the protein phosphatase 1 catalytic subunit alpha (PP1α) has been suggested (Doerks et al., 2002) and murine Pp1γ2 has been identified to play a crucial role in the morphogenesis and motility of adult spermatozoa (Chakrabarti et al., 2007; Fardilha et al., 2011). Therefore, we next wanted to investigate, if Ccdc181 also interacts with other catalytic subunits of Pp1, especially with Pp1γ2, by yeast two hybrid. As shown in Fig. 10, Ccdc181 and Ccdc181-D1 were able to interact with Pp1α, Pp1β, Pp1γ1 and Pp1γ2. In contrast no interaction with the different catalytic subunits of Pp1 was observed for Ccdc181-D2 and Ccdc181-D3. This interaction of Ccdc181 and Pp1γ2 further strengthens our hypothesis that Ccdc181 might play an important role in ciliary motility.
Figure 10. Ccdc181 interacts with catalytic subunits of Pp1 by yeast two hybrid.
Yeast cells harboring different combinations of Ccdc181 constructs in pGADT7 or empty pGADT7 and catalytic subunits of Pp1 in pGBKT7 or empty pGBKT7 as indicated were grown on medium lacking leucine and tryptophan (double dropout) to verify co-transformation. Interaction was assessed by analyzing yeast growth on medium lacking adenine, histidine, leucine and tryptophan (quadruple dropout). Ccdc181 and Ccdc181-D1 were shown to interact with the indicated catalytic subunits of Pp1.
Discussion
We previously demonstrated that the disruption of Hook1 is the underlying cause of the azh (abnormal spermatozoon head shape) phenotype in mice. In mice carrying the mutation azh in a homozygous state the spermatozoa display abnormal head morphology and often tail abnormalities resulting in coiled sperm tails or in the decapitation of the sperm head from the flagellum. However, it seems unlikely that Hook1 acts as a structural component of the spermatozoa, as it cannot be detected in mature mouse spermatozoa (Mendoza-Lujambio et al., 2002). Rather it can be assumed that these abnormalities in mature sperms are indirectly caused by the loss of Hook1 function causing a dislocation of proteins during spermatid differentiation. One of the multiple specialized organelles proposed to ensure correct maturation of spermatozoa is the manchette. This specialized structure consists of laterally associated microtubules appearing transiently during spermiogenesis and is thought to play a role in nuclear shaping and intramanchette transport (IMT) processes, a putatively affected process by the azh mutation (Kierszenbaum, 2002; Toshimichi Yoshida, 1994).
We assume that Hook1 mediates cargo transport along the microtubules of the manchette, as it has already been shown that Hook1 interacts with microtubules of the manchette through its N-terminal microtubule-binding domain and contains a putative organelle-binding domain at its C-terminus (Mendoza-Lujambio et al., 2002; Walenta et al., 2001). To identify putative Hook1 interacting proteins that might serve as Hook1 cargo proteins in murine spermiogenesis we performed a yeast two hybrid screen by which several different putative interacting proteins were found. One of the identified cDNA fragments revealed a strong similarity to the coiled-coil domain containing protein 181 (Ccdc181). Ccdc181 belongs to the large family of polypeptides displaying a coiled-coil domain. However, computational search revealed no significant similarity to other proteins of the coiled-coil domain containing protein family, but showed that Ccdc181 is evolutionary conserved. Additionally, Ccdc181 was shown to be a putative ciliary protein (McClintock et al., 2008). Our results support this finding and indicate that Ccdc181 is a structural component of the axonemal structure of sperm flagella as well as motile cilia. Expression analyses identified low levels of ubiquitously expressed Ccdc181 mRNA, although the strongest expression was observed in testis. Furthermore, elevated levels of Ccdc181 transcripts could be detected in several tissues expressing motile cilia supporting its putative function as a ciliary protein. We used specific antibodies to localize Ccdc181 in ciliary and flagellar structures and found Ccdc181 distributed along the sperm flagellum whereas localization of Ccdc181 was restricted to the basal part of motile cilia. Interestingly, we could not detect Ccdc181 in the non-motile single cilium of NIH 3T3 cells indicating that Ccdc181 is not a component of the basic axonemal structure that is highly similar in motile as well as in non-motile cilia (Satir and Christensen, 2007). Rather it seems that Ccdc181 is involved in the generation of ciliary and flagellar motility although its function in this process is unclear. This assumption is supported by the observation that Ccdc181 can interact with different isoforms of the catalytic subunits of the phosphoprotein phosphatase 1 (PP1). PP1 is a major serine-threonine phosphatase that is ubiquitously distributed and is involved in a broad range of cellular functions, including cell cycle progression (Korrodi-Gregorio et al., 2014). The PP1 family consists of four mainly homologues catalytic subunits, the PP1α, PP1β, PP1γ1 and PP1γ2. Previous findings suggested that PP1γ2 is predominantly expressed in testicular tissues and plays an important role in regulating the motility of spermatozoa (Fardilha et al., 2011). Interestingly, a knock-down of Pp1γ2 leads to male infertility due to immotile spermatozoa and cannot be rescued by the other members of the Pp1 family, whereas in other tissues the loss of a Pp1 subunit can be substituted by the remaining Pp1 subunits (Fardilha et al., 2011; Sinha et al., 2013). As Pp1α has been identified as a putative Ccdc181 binding protein (Doerks et al., 2002) and the high overall similarity of the Pp1 catalytic subunits, we tested whether Ccdc181 is also capable of binding to the other Pp1 catalytic subunits. Our results indicate that the N-terminal part of Ccdc181 interacts with Pp1α, Pp1β, Pp1γ1 and Pp1γ2. Additionally we observed that the C-terminal region (AA 400-450) of Ccdc181 interacts with microtubules and Hook1. This raises the hypothesis that Ccdc181 acts as a linker protein for Pp1 to tack the phosphatase to the axonemal structure of cilia and flagella. Pp1 does not only control motility in spermatozoa but has been recently identified as a putative regulator of motility in mammalian airway cilia (Price et al., 2015). Taken together our results suggest that Ccdc181 via its interaction with Pp1 might play an important role in the generation and regulation of flagellar as well as ciliary motility. We found that the C-terminal region of Ccdc181 is responsible for the binding to microtubules. Interestingly, this domain is also used for the interaction with Hook1 suggesting that Ccdc181 exclusively pbinds to either Hook1 or microtubules. In contrast, the necessary amino acids of Hook1 needed for interaction with Ccdc181 do not overlap with the putative microtubule binding domain of Hook1. Although not yet proven experimentally we assume that Ccdc181 does not bind directly to the microtubules of the manchette in spermatids but rather is linked to the manchette by Hook1 for intramanchette transportation to the axoneme of the growing sperm flagella and in this context avoid any unwanted binding of Ccdc181 to the microtubules of the manchette. Additionally, we demonstrated in-vitro that Ccdc42 is a putative Hook1 interacting protein (Fig. S6). Interestingly, Ccdc42 has recently been reported as a flagellar protein which plays a crucial role in male germ cell development (Pasek et al., 2016), strengthening our hypothesis that Hook1 acts as a cargo linker in intra-manchette transport processes for different proteins such as Ccdc42 and Ccdc181. In meiotic stages of male germ cell differentiation we detected Ccdc181 staining in the cytoplasm as well as at the microtubules of the spindle apparatus indicating an involvement of Ccdc181 in the process of meiotic division. Recently, Ccdc181 was identified as interacting partner of the RING domain-containing protein CCNB1IP1 (Cyclin B1 Interacting Protein 1), which is a putative ubiquitin E3 ligase that seems to be essential for chiasmata formation during meiosis (Strong and Schimenti, 2010). Moreover, CCNB1IP1 has also been identified as a Hook1 interacting protein (Strong and Schimenti, 2010). Further experiments have to be performed to elucidate the impact of these different interactions on each other and their role in spermatogenesis.
Conclusions
We report the molecular characterization of a new member of the coiled-coil domain containing protein family encoded by the murine Ccdc181 gene. Ccdc181 is a Hook1 interacting protein, supporting the hypothesis that Hook1 acts as a nonspecific linker protein for sperm components to the intramanchette transport system in post-meiotic male germ cells. Ccdc181 seems to be able to bind tightly to axonemal structures as they are found in cilia and sperm flagella. Therefore, a direct binding of Ccdc181 to the microtubular structures of the manchette in the spermatid might result in a dislocation of Ccdc181 within the elongating spermatid and has to be avoided. Our data indicate that interaction between Hook1 and Ccdc181 blocks the putative tubulin-binding domain of Ccdc181 in the developing spermatid. Although the molecular function of Ccdc181 is yet unknown, a function of Ccdc181 as a linker protein between microtubules of the axoneme or meiotic spindle apparatus and other polypeptides such as protein phosphatase 1 or the cyclin B1 interacting protein 1 can be assumed. Taken together, our results support Ccdc181 as a structural component of cilia and sperm flagella and sustain the human CCDC181 gene is a good candidate for patients suffering from a disturbed ciliary motility.
Supplementary Material
Living NIH 3T3 cells expressing the controls GFP-only, DsRed-only, GFP and DsRed as well as the GFP-DsRed fusion protein were analyzed on a LSR Fortessa flow cytometer. Double positive cells were gated (panel 1) while false positive FRET signals resulting from DsRed excitation by the 488 nm laser were excluded (panel 2). For the remaining cells a gate was set up to exclude FRET negative signals from cells co-expressing GFP and DsRed. Therefore, only FRET positive signals from cells expressing the GFP-DsRed fusion protein were counted (panel 3).
(A) A yeast two hybrid assay using different constructs of Ccdc181 in pGADT7 and Hook2 or Hook3 in pGBKT7 showed an interaction of Ccdc181 with Hook2 and Hook3 on plates lacking adenine, histidine, leucine and tryptophan. (B) Putative homodimerization of Ccdc181 was shown by yeast two hybrid using indicated combinations of Ccdc181 constructs in pGADT7 and pGBKT7.
Ccdc181 expression was analyzed by RT-PCR and Northernblot. For RT-PCR expression of Gapdh was used as control, whereas for Northern blot 18S and 28S rRNA served as controls for RNA integrity. (A, B) Analysis of Ccdc181 expression in indicated mouse tissues, revealed a predominant testicular expression. (C, D) In different mouse mutants, no Ccdc181 expression was observed in mutants lacking either germ cells (W/Wv) or haploid spermatids (Tfm/y). (E) Determination of Ccdc181 expression in postnatal testicular stages showed an increase of Ccdc181 expression during late stages of spermatogenesis.
Indirect immunofluorescence staining of prepared germ cell suspensions from adult C57BL/6 mice was performed using directly labeled polyclonal rabbit anti-Ccdc181 (Alexa Flour 488, green) and anti-Hook1 (Alexa Fluor 555, red). Acrosomes were visualized by peanut-lectin (white) and nuclei were stained with DAPI (blue). (a-d) In spermatocytes only a diffuse staining of Ccdc181 and Hook1 was observed. (e-l) In post-meiotic haploid male germ cell stages Ccdc181 and Hook1 both co-localize with the microtubular manchettes. Scale bars: 5 μm.
Localization of Ccdc181 in sections of indicated tissues was analyzed by immunohistochemical staining. Ccdc181 staining was restricted to ciliated cells of the epithelial layer (arrows) of oviduct (a, b), ventricles of the brain (d, e), trachea (g, h) and bronchi and bronchioles of the lung (j, k). In controls Ccdc181 antibody staining was omitted (c, f, i, l). Scale bars: 50 μm.
Interaction between Ccdc42 and Hook1 was verified using FACS based FRET analysis on a CytoFlex S. (A) Representative FACS-plots showing the amount of FRET positive cells in living NIH 3T3 cells co-expressing the indicated GFP and DsRed fusion proteins. FRET positive cells within the gates (orange box) are displayed in green, while FRET negative cells outside the gates are displayed in red. Numbers give total percentages of cells within the FRET gate. The non-interacting proteins GFP and DsRed were used as a negative control, whereas Hook1’s ability to form homodimers was used as a positive control. (B) Quantification of FRET positive cells using mean values and standard error of the mean for the total amount of FRET positive cells from three independent experiments, that were analyzed as depicted in (A). P ≤ 0,0001.
Acknowledgements
We thank Dr. Sabine Rauscher and Dr. Marion Gröger of the CEMM Imaging Core Facility for their technical assistance on the Zeiss LSM780 and are grateful to Dr. Andreas Spittler for advice and help by establishing the FACS-FRET method at the CEMM Cytometry Core Facility. This work was funded by the FWF grant P22761-B13.
Footnotes
Conflict of interests
The authors declare that they have no conflicts of interest with the contents of this article.
Contributors
TS performed the experiments and collected the data. BP helped generating the constructs and was responsible for cell culture maintenance. TS, BP and JN drafted the manuscript. PG supported us with tissues from various mouse mutants. JN formulated the original problem. JS assisted with data analysis and provided helpful feedback and guidance. All authors read and approved the final manuscript.
References
- Banning C, Votteler J, Hoffmann D, Koppensteiner H, Warmer M, Reimer R, Kirchhoff F, Schubert U, Hauber J, Schindler M. A flow cytometry-based FRET assay to identify and analyse protein-protein interactions in living cells. PloS one. 2010;5:e9344. doi: 10.1371/journal.pone.0009344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron Gaillard CL, Pallesi-Pocachard E, Massey-Harroche D, Richard F, Arsanto JP, Chauvin JP, Lecine P, Kramer H, Borg JP, Le Bivic A. Hook2 is involved in the morphogenesis of the primary cilium. Molecular biology of the cell. 2011;22:4549–4562. doi: 10.1091/mbc.E11-05-0405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellve AR, Cavicchia JC, Millette CF, O'Brien DA, Bhatnagar YM, Dym M. Spermatogenic cells of the prepuberal mouse. Isolation and morphological characterization. The Journal of cell biology. 1977;74:68–85. doi: 10.1083/jcb.74.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti R, Kline D, Lu J, Orth J, Pilder S, Vijayaraghavan S. Analysis of Ppp1cc-null mice suggests a role for PP1gamma2 in sperm morphogenesis. Biology of reproduction. 2007;76:992–1001. doi: 10.1095/biolreprod.106.058610. [DOI] [PubMed] [Google Scholar]
- Chubb C. Oligotriche and Quaking Gene Mutations Phenotypic Effects on Mouse Spermatogenesis and Testicular Steroidogenesis. Journal of andrology. 1992;13:312–317. [PubMed] [Google Scholar]
- Doerks T, Copley RR, Schultz J, Ponting CP, Bork P. Systematic identification of novel protein domain families associated with nuclear functions. Genome research. 2002;12:47–56. doi: 10.1101/gr.203201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fardilha M, Esteves SL, Korrodi-Gregorio L, Pelech S, da Cruz ESOA, da Cruz ESE. Protein phosphatase 1 complexes modulate sperm motility and present novel targets for male infertility. Molecular human reproduction. 2011;17:466–477. doi: 10.1093/molehr/gar004. [DOI] [PubMed] [Google Scholar]
- Gietz RD. Yeast Transformation by the LiAc/SS Carrier DNA/PEG Method. In: Smith JS, Burke DJ, editors. Yeast Genetics. Springer; New York: 2014. pp. 1–12. [Google Scholar]
- Goujon M, McWilliam H, Li W, Valentin F, Squizzato S, Paern J, Lopez R. A new bioinformatics analysis tools framework at EMBL–EBI. Nucleic acids research. 2010;38:W695–W699. doi: 10.1093/nar/gkq313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haldrup C, Mundbjerg K, Vestergaard EM, Lamy P, Wild P, Schulz WA, Arsov C, Visakorpi T, Borre M, Hoyer S, Orntoft TF, et al. DNA methylation signatures for prediction of biochemical recurrence after radical prostatectomy of clinically localized prostate cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2013;31:3250–3258. doi: 10.1200/JCO.2012.47.1847. [DOI] [PubMed] [Google Scholar]
- Hayasaka S, Terada Y, Suzuki K, Murakawa H, Tachibana I, Sankai T, Murakami T, Yaegashi N, Okamura K. Intramanchette transport during primate spermiogenesis: expression of dynein, myosin Va, motor recruiter myosin Va, VIIa-Rab27a/b interacting protein, and Rab27b in the manchette during human and monkey spermiogenesis. Asian journal of andrology. 2008;10:561–568. doi: 10.1111/j.1745-7262.2008.00392.x. [DOI] [PubMed] [Google Scholar]
- He M, Subramanian R, Bangs F, Omelchenko T, Liem KF, Jr, Kapoor TM, Anderson KV. The kinesin-4 protein Kif7 regulates mammalian Hedgehog signalling by organizing the cilium tip compartment. Nature cell biology. 2014;16:663–672. doi: 10.1038/ncb2988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kierszenbaum AL. Intramanchette transport (IMT): managing the making of the spermatid head, centrosome, and tail. Molecular reproduction and development. 2002;63:1–4. doi: 10.1002/mrd.10179. [DOI] [PubMed] [Google Scholar]
- Kierszenbaum AL, Tres LL. The acrosome-acroplaxome-manchette complex and the shaping of the spermatid head. Arch Histol Cytol. 2004;67:271–284. doi: 10.1679/aohc.67.271. [DOI] [PubMed] [Google Scholar]
- Korrodi-Gregorio L, Esteves SL, Fardilha M. Protein phosphatase 1 catalytic isoforms: specificity toward interacting proteins. Translational research : the journal of laboratory and clinical medicine. 2014;164:366–391. doi: 10.1016/j.trsl.2014.07.001. [DOI] [PubMed] [Google Scholar]
- Kramer H, Phistry M. Genetic analysis of hook, a gene required for endocytic trafficking in drosophila. Genetics. 1999;151:675–684. doi: 10.1093/genetics/151.2.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lupas A, Van Dyke M, Stock J. Predicting coiled coils from protein sequences. Science (New York, N.Y.) 1991;252:1162–1164. doi: 10.1126/science.252.5009.1162. [DOI] [PubMed] [Google Scholar]
- Lyon MF, Glenister PH, Lynn Lamoreux M. Normal spermatozoa from androgen-resistant germ cells of chimaeric mice and the role of androgen in spermatogenesis. Nature. 1975;258:620–622. doi: 10.1038/258620a0. [DOI] [PubMed] [Google Scholar]
- Maldonado-Baez L, Cole NB, Kramer H, Donaldson JG. Microtubule-dependent endosomal sorting of clathrin-independent cargo by Hook1. The Journal of cell biology. 2013;201:233–247. doi: 10.1083/jcb.201208172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClintock TS, Glasser CE, Bose SC, Bergman DA. Tissue expression patterns identify mouse cilia genes. Physiological genomics. 2008;32:198–206. doi: 10.1152/physiolgenomics.00128.2007. [DOI] [PubMed] [Google Scholar]
- Meistrich ML, Trostle-Weige PK, Russell LD. Abnormal manchette development in spermatids of azh/azh mutant mice. The American journal of anatomy. 1990;188:74–86. doi: 10.1002/aja.1001880109. [DOI] [PubMed] [Google Scholar]
- Mendoza-Lujambio I, Burfeind P, Dixkens C, Meinhardt A, Hoyer-Fender S, Engel W, Neesen J. The Hook1 gene is non-functional in the abnormal spermatozoon head shape (azh) mutant mouse. Human molecular genetics. 2002;11:1647–1658. doi: 10.1093/hmg/11.14.1647. [DOI] [PubMed] [Google Scholar]
- Mochida K, Tres LL, Kierszenbaum AL. Structural and biochemical features of fractionated spermatid manchettes and sperm axonemes of the azh/azh mutant mouse. Molecular reproduction and development. 1999;52:434–444. doi: 10.1002/(SICI)1098-2795(199904)52:4<434::AID-MRD13>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Ohta H, Tohda A, Nishimune Y. Proliferation and differentiation of spermatogonial stem cells in the w/wv mutant mouse testis. Biology of reproduction. 2003;69:1815–1821. doi: 10.1095/biolreprod.103.019323. [DOI] [PubMed] [Google Scholar]
- Pasek RC, Malarkey E, Berbari NF, Sharma N, Kesterson RA, Tres LL, Kierszenbaum AL, Yoder BK. Coiled-coil domain containing 42 (Ccdc42) is necessary for proper sperm development and male fertility in the mouse. Developmental biology. 2016;412:208–218. doi: 10.1016/j.ydbio.2016.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price ME, Pavlik JA, Sisson JH, Wyatt TA. Inhibition of protein phosphatase 1 reverses alcohol-induced ciliary dysfunction. Am J Physiol Lung Cell Mol Physiol. 2015;308:L577–585. doi: 10.1152/ajplung.00336.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satir P, Christensen ST. Overview of structure and function of mammalian cilia. Annual review of physiology. 2007;69:377–400. doi: 10.1146/annurev.physiol.69.040705.141236. [DOI] [PubMed] [Google Scholar]
- Sievers F, Wilm A, Dineen D, Gibson TJ, Karplus K, Li W, Lopez R, McWilliam H, Remmert M, Söding J, Thompson JD, et al. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Molecular Systems Biology. 2011;7 doi: 10.1038/msb.2011.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha N, Puri P, Nairn AC, Vijayaraghavan S. Selective ablation of Ppp1cc gene in testicular germ cells causes oligo-teratozoospermia and infertility in mice. Biology of reproduction. 2013;89:128. doi: 10.1095/biolreprod.113.110239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strong ER, Schimenti JC. Evidence Implicating CCNB1IP1, a RING Domain-Containing Protein Required for Meiotic Crossing Over in Mice, as an E3 SUMO Ligase. Genes. 2010;1:440–451. doi: 10.3390/genes1030440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toshimichi Yoshida SOI, Kyoko Imanaka-Yoshida, Kosaku Izutsu. Association of cytoplasmic dynein with manchette microtubules and spermatid nuclear envelope during spermiogenesis in rats. Journal of cell science. 1994;107:9. doi: 10.1242/jcs.107.3.625. [DOI] [PubMed] [Google Scholar]
- Walenta JH, Didier AJ, Liu X, Kramer H. The Golgi-associated hook3 protein is a member of a novel family of microtubule-binding proteins. The Journal of cell biology. 2001;152:923–934. doi: 10.1083/jcb.152.5.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Living NIH 3T3 cells expressing the controls GFP-only, DsRed-only, GFP and DsRed as well as the GFP-DsRed fusion protein were analyzed on a LSR Fortessa flow cytometer. Double positive cells were gated (panel 1) while false positive FRET signals resulting from DsRed excitation by the 488 nm laser were excluded (panel 2). For the remaining cells a gate was set up to exclude FRET negative signals from cells co-expressing GFP and DsRed. Therefore, only FRET positive signals from cells expressing the GFP-DsRed fusion protein were counted (panel 3).
(A) A yeast two hybrid assay using different constructs of Ccdc181 in pGADT7 and Hook2 or Hook3 in pGBKT7 showed an interaction of Ccdc181 with Hook2 and Hook3 on plates lacking adenine, histidine, leucine and tryptophan. (B) Putative homodimerization of Ccdc181 was shown by yeast two hybrid using indicated combinations of Ccdc181 constructs in pGADT7 and pGBKT7.
Ccdc181 expression was analyzed by RT-PCR and Northernblot. For RT-PCR expression of Gapdh was used as control, whereas for Northern blot 18S and 28S rRNA served as controls for RNA integrity. (A, B) Analysis of Ccdc181 expression in indicated mouse tissues, revealed a predominant testicular expression. (C, D) In different mouse mutants, no Ccdc181 expression was observed in mutants lacking either germ cells (W/Wv) or haploid spermatids (Tfm/y). (E) Determination of Ccdc181 expression in postnatal testicular stages showed an increase of Ccdc181 expression during late stages of spermatogenesis.
Indirect immunofluorescence staining of prepared germ cell suspensions from adult C57BL/6 mice was performed using directly labeled polyclonal rabbit anti-Ccdc181 (Alexa Flour 488, green) and anti-Hook1 (Alexa Fluor 555, red). Acrosomes were visualized by peanut-lectin (white) and nuclei were stained with DAPI (blue). (a-d) In spermatocytes only a diffuse staining of Ccdc181 and Hook1 was observed. (e-l) In post-meiotic haploid male germ cell stages Ccdc181 and Hook1 both co-localize with the microtubular manchettes. Scale bars: 5 μm.
Localization of Ccdc181 in sections of indicated tissues was analyzed by immunohistochemical staining. Ccdc181 staining was restricted to ciliated cells of the epithelial layer (arrows) of oviduct (a, b), ventricles of the brain (d, e), trachea (g, h) and bronchi and bronchioles of the lung (j, k). In controls Ccdc181 antibody staining was omitted (c, f, i, l). Scale bars: 50 μm.
Interaction between Ccdc42 and Hook1 was verified using FACS based FRET analysis on a CytoFlex S. (A) Representative FACS-plots showing the amount of FRET positive cells in living NIH 3T3 cells co-expressing the indicated GFP and DsRed fusion proteins. FRET positive cells within the gates (orange box) are displayed in green, while FRET negative cells outside the gates are displayed in red. Numbers give total percentages of cells within the FRET gate. The non-interacting proteins GFP and DsRed were used as a negative control, whereas Hook1’s ability to form homodimers was used as a positive control. (B) Quantification of FRET positive cells using mean values and standard error of the mean for the total amount of FRET positive cells from three independent experiments, that were analyzed as depicted in (A). P ≤ 0,0001.