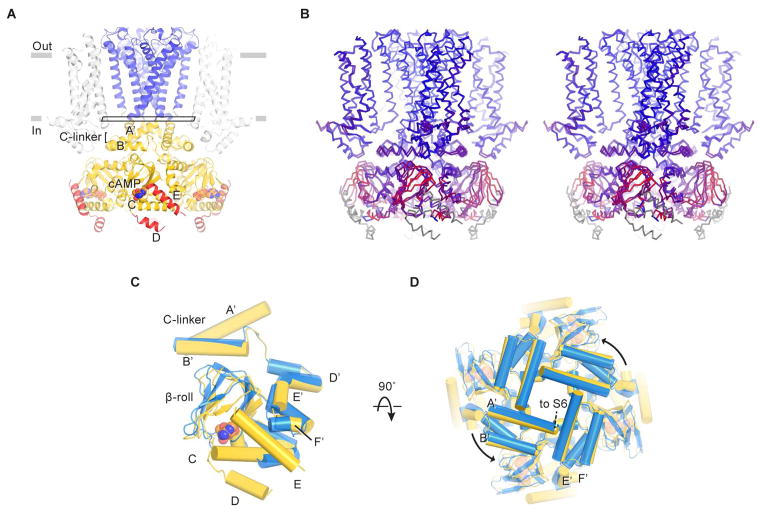
Figure 7. Cyclic AMP modulation of the HCN1 channel.
(A) Structure of the channel in the cAMP-bound state. D- and E-helices are colored red. The rectangle near the gate indicates the plane of the “slice” used for the view in (D).
(B) Stereo view of the superposition of ligand-free and cAMP-bound structures based on Cα atoms of the pore helices. The channel is colored on a red-to-blue spectrum according to the displacement of Cα atoms between the two structures. Blue color represents minimal displacements and red color represents displacements up to 4.5 Å. Gray color indicates residues only present in one structure and thus are not aligned.
(C and D) Superposition of C-linkers and CNBDs in the ligand-free (blue) and cAMP-bound states (yellow). Only single subunits are shown in (C) for clarity. (D) is viewed from the extracellular side.
See also Figures S4 and S7.