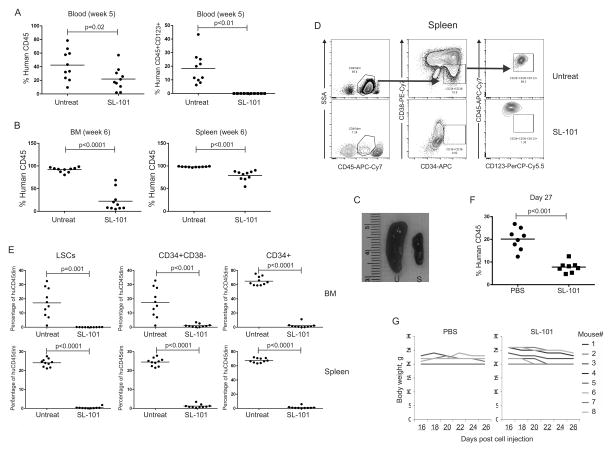
Figure 5. SL-101 targets AML leukemia-initiating cells in vivo.
The primary AML patient sample 11 (4030094) was left untreated or pre-treated with SL-101 (1.0 μg/mL) overnight prior to transplantation via intravenous injection into NSG mice (1×106 viable cells for each mouse). (A) Engraftment of human AML cells was measured by flow cytometry in blood by using anti-human CD45 antibody at week 5 after injection (left). CD123+ cells were gated on the human CD45+ fraction (right). (B) At week 6, all mice were sacrificed and engraftment of human AML was measured in both bone marrow (BM) and spleen. (C) Representative spleens from untreated (U) and SL-101-treated (S) mice are shown. (D) The gating scheme is shown for the CD45dimCD34+CD38−CD123+ LSC-enriched population in untreated and SL-101–treated mice. (E) Percentages of LSCe in human CD45+ cells were determined in both bone marrow and spleen by flow cytometry. AML11 cells (0.6×106) were injected into NSGS) mice that were treated intravenously with PBS or SL-101 at 0.1 mg/kg every other day for 6 doses, after confirming human CD45 engraftment. (F) Leukemia burden was measured after treatment (G) Body weight was monitored over treatment.