Figure 8.
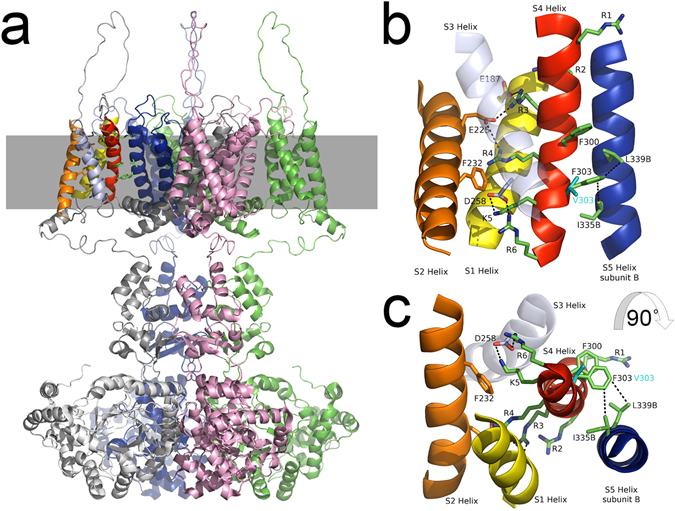
Model structure of the human Kv1.1 potassium channel in the open-state. (a) The structure of the tetrameric channel is shown with each of the four polypeptide subunits colored overall grey, green, pink and blue. The position of the membrane is illustrated by a grey band and the brightly colored helices in the top left of the figure are shown in the expanded Figures b and c. (b) The membrane spanning region of interest depicting four helices of one subunit (S1 yellow, S2 orange, S3 lavender and S4 red) and one helix from a neighboring subunit (S5B, blue) are shown. Helix S3 lies in front of the other helices and is rendered transparent for clarity. Significant side chains are drawn in stick representations and binding partners indicated by black dotted lines between them. These include R3-E187, R3-E225, R4-E225, K5-D258, R6-D258, F303-L339B and F303-I335B. The predicted position of the V303 mutant side chain is also shown. (c) Illustrates the same structure as depicted in B rotated ninety degrees towards the observer. The stacking of F300 and F303 is well illustrated in this figure.
