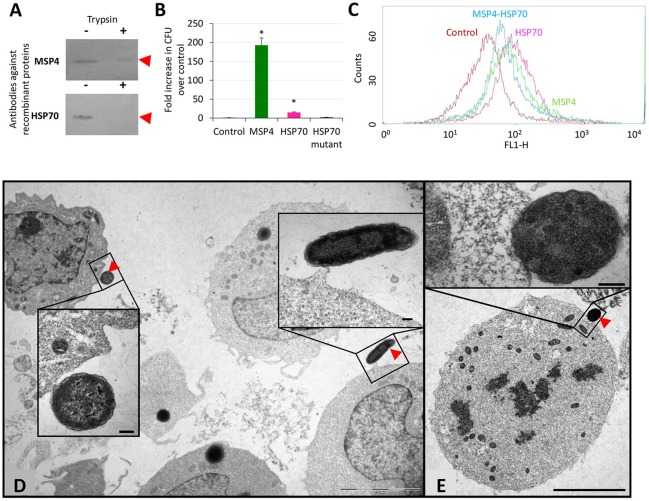
Figure 1.
Role of A. phagocytophilum MSP4 and HSP70 proteins in interactions with HL60 human cells. (A) A. phagocytophilum (NY18) purified from infected HL60 human cells were mock treated (−) or surface digested with trypsin (+) and 10 μg protein loaded onto polyacrylamide gels for Western blot analysis using rabbit antibodies produced against recombinant proteins. These experiments have been repeated three times with similar results. (B) E. coli strains were grown and induced for the production of recombinant proteins. E. coli cells transformed with expression vector alone were used as negative control (Control). Bacteria adhesion to human HL60 cells was quantitated as the number of colony forming units (CFU) recovered from each test and compared to the control values by Chi2 test. Asterisks denote statistical significant differences between CFU recovered from E. coli transformed with MSP4 or HSP70 and the control (P < 0.001; N = 2 replicates per treatment). (C) HL-60 cells were incubated with A. phagocytophilum human NY18 isolate in the presence of recombinant MSP4, HSP70 and their combination. HL60 cells incubated with PBS served as negative controls. After washing to remove unbound bacteria and proteins, host cells were incubated for 48 h and binding of recombinant proteins to human host cells was analyzed by flow cytometry. The viable cell population was gated according to forward scatter and side scatter parameters. (D,E) Representative images of the adhesion of recombinant E. coli producing membrane exposed A. phagocytophilum proteins to human HL60 cells. (D) E. coli producing MSP4 or (E) HSP70 were incubated with HL60 cells and revealed by TEM to show adhesion to human cells (arrows). Cells incubated with control bacteria did not show adhesion to HL60 cells. Details of both interacting cells are shown in insets. Scale bars: 5 μm (D,E) and 200 nm (insets).