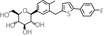
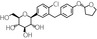
Table 1.
Chemical and pharmacological characteristics of SGLT2 inhibitors available in Japan
| Generic name | Ipragliflozin | Dapagliflozin | Luseogliflozin | Tofogliflozin | Canagliflozin | Empagliflozin |
|---|---|---|---|---|---|---|
| Structural formula |
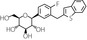
|
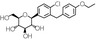
|
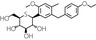
|
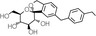
|
|
|
| Initial marketing | 2014 April | 2014 May | 2014 May | 2014 May | 2014 September | 2015 Ferbruary |
| Dosage and administration | 50 mg once daily (to 100 mg once daily) | 5 mg once daily (to 10 mg once daily) | 2.5 mg once daily (to 5 mg once daily) | 20 mg once daily | 100 mg once daily | 10 mg once daily (to 25 mg once daily) |
| SGLT2/SGLT1 | 860 | 610 | 1,770 | 2,900 | 290 | 2,680–5,000 |
| Half‐life (h) | 11.71 | 12.1 | 11.2 | 5.4 | 10.6 | 9.88–11.7 |
| Bioavailability | 90.20% | 78% | 90% | 97.50% | 65% | NA |
| Protein binding | 94.6–96.5% | 91% | 96.0–96.3% | 82.3–82.6% | 99% | 86.20% |
| Metabolism | UGT2B7 | UGT1A9 | CYP3A4/5, 4A11, 4F2, 4F3B, UGT1A1 | CYP3A4/5, 4A11, 4F3B | UGT1A9, 2B4, 3A4, CYP2D6 | UGT2B7, 1A3, 1A9 |
| Excretion |
Urinary ex. 67.9% Fecal ex 32.7% |
Urinary ex. 75.0% Fecal ex. 21.0% |
Urinary ex. 44.2% Fecal ex. 50.0% |
Urinary ex. 76.2% Fecal ex. 21.4% |
Urinary ex. 32.5% Fecal ex. 60.4% |
Urinary ex. 54.4% Fecal ex. 41.2% |
CYP, cytochrome P450 superfamily; N/A, not available; UGT, UDP‐glucosyltransferase.
