Abstract
Morpholino oligomers (MOs) are antisense molecules designed for sequence-specific binding of target mRNA. In bacteria, inhibition is hypothesized to occur by preventing translation initiation. Cell- penetrating peptides may be conjugated to the 5′- or 3′-termini of an MO to enhance cellular entry and therefore inhibition. Here we describe the three standard microbiological assays to assess in vitro antibacterial MO efficacy.
Keywords: Antisense, Morpholino oligomers (MOs), Minimum inhibitory concentration (MIC), Minimum bactericidal concentration (MBC), Checkerboard synergy
1 Introduction
Assessing the susceptibility or resistance of bacteria to a particular antimicrobial treatment has been done for more than 100 years. In modern times, this practice has been standardized by the Clinical and Laboratory Standards Institute (CLSI) to allow clinical labs across world to produce accurate information for the health care community. These CLSI guidelines have defined the minimal inhibitory concentration (MIC) as the lowest concentration of an antimicrobial agent that prevents visible growth of a microorganism in an agar or broth dilution susceptibility test [1]. Clinically, these assays are used to assess whether a specific bacterium is resistant or sensitive to therapeutic antimicrobials. The MIC method described here is based upon the CLSI broth microdilution method.
MICs assays assess growth inhibition but are not themselves indicative of bacterial death. However, in addition to the MIC method, the CLSI has developed guidelines for testing the minimal bactericidal concentration (MBC), which is defined as at least a 99.9% (≥3-log) reduction in bacterial burden [2]. Again, the MBC method described here is based upon the CLSI bactericidal method.
Increasingly, bacterial inhibition using multiple antimicrobials has been used as a way to determine the potential interactions (synergistic, additive, or antagonistic) of two agents. Reasons for assessing these interactions include the increasing incidence of multidrug-resistant bacteria. Although not adequately tested in clinical trials, using multiple therapeutics has several proposed benefits and drawbacks (reviewed in ref. [3]). Proposed benefits include: (a) broader coverage during empiric therapy, (b) synergistic or additive interactions between compounds, and (c) inhibiting multiple targets at once might decrease the emergence of resistance; while proposed drawbacks include: (a) increased toxicities, (b) potential for drug–drug interactions, and (c) increased costs [3]. The synergy assay described here is a modified MIC assay in which each antibacterial agent is diluted to generate every twofold dose combination. The resulting growth inhibition of their combinations is assessed to determine the interaction.
MOs are conjugated to cell-penetrating peptides to enhance cellular entry [4, 5]. We have found that the most efficient cell- penetrating peptide depends on the organism. For example, the most efficient peptide in Burkholderia to date is (RFF)3RXB while in Acinetobacter and Pseudomonas to date it is (RXR)4XB [6–8]. Review of the literature may provide insight for novel organisms, otherwise empiric testing will be required. However, even with cell-penetrating peptides MOs have trouble penetrating the cell membranes of Pseudomonas aeruginosa. To circumvent this entry defect we have found that sub-inhibitory concentrations of the cyclic portion of polymyxin B, polymyxin B nonapeptide (PMBN), enhance entry of MOs. For example in P. aeruginosa PAO1 the MIC of PMBN is >16 μg/mL and 2 μg/mL of PMBN can enhance MO MICs by four- to eight-fold [8].
When testing essential genes we have found that growth media can play a significant role. Standard CLSI guidelines for MIC testing are in cation-adjusted Mueller-Hinton II medium, but careful consideration should be given to experimental conditions most similar to the physiologic environment that would be encountered by the pathogen and antisense molecule in a therapeutic setting. For example, in P. aeruginosa the MIC in a minimal media was up to eightfold lower than in MHII [8]. It should also be noted that different media might be required based on the auxotrophic requirements of different organisms.
Finally, the procedures described below are specifically for aerobic, nonfastidious organisms that grow optimally at 37 °C. Alterations in culture conditions will be required for organisms that do not fit these parameters.
2 Materials
Morpholino oligomers are usually supplied as lyophilisates and can be stored at −20 °C. Stocks should be dissolved in ultrapure water (≥18 MΩ cm) and stored at 4 °C. While the MO itself is quite stable, the cell-penetrating peptides are much less stable. Therefore they should be kept as concentrated stocks, and all solutions should be kept refrigerated or on ice while setting up any assays.
2.1 MIC Assay
1 mM morpholino oligomer (MO) stock.
96-well plate spectrophotometer capable of reading optical density at 600 nm (see Note 1).
96-well polystyrene plates (see Note 2).
Gas permeable plate sealing film (see Note 3).
Mueller-Hinton II (MHII) broth (cation-adjusted).
MHII agar (alternate agar may be substituted).
Phosphate Buffered Saline (PBS).
2.2 MBC Assay
MIC Assay materials.
2.3 Synergy Assay
MIC Assay materials.
Antibiotic stock solutions prepared as per manufacturer.
3 Methods
3.1 Minimum Inhibitory Concentration (MIC) Assay
The volumes described are sufficient for one 96-well plate, capable of testing six MOs in duplicate (experimental replicates).
Bacterial colonies should be freshly obtained from −80 °C laboratory stocks by streaking onto an appropriate agar plate and incubating overnight at 37 °C.
Inoculate 3 mL of MHII and incubate 16–18 h at 37 °C on a 220 rpm shaker (see Note 4). If the culture will not be used immediately, place on ice.
Dilute the overnight culture in PBS until the optical density at 600 nm (OD600) is between 0.07 and 0.1. This is roughly equivalent to 1 × 108 CFU/mL (see Note 5).
Add 60 μL of the OD600 stock to 12 mL of MHII to attain 5 × 105 CFU/mL. Vortex vigorously.
Serially dilute and plate this stock to determine CFU/mL of the inoculum. (see Note 6)
Aliquot 442.8 μL of the 5 × 105 CFU/mL stock into individual tubes and add 7.2 μL of each 1 mM MO stock to be tested. Vortex. This results in a final concentration of 16 μM, and is typically the highest preliminary concentration tested (see Note 7).
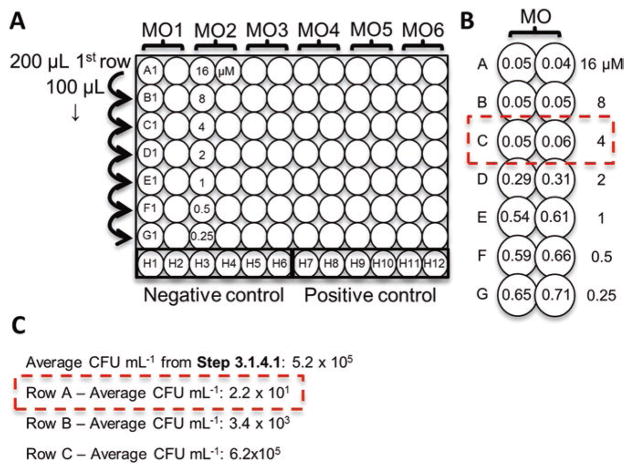
Add 200 μL of each MO stock from step 6 in duplicate wells to row A of a 96-well plate (see Fig. 1a).
Aliquot 100 μL of the 5 × 105 CFU/mL to rows B-G and in wells H7–12 (positive control). Aliquot 100 μL of MHII to wells H1–6 (negative control).
Transfer 100 μL from row A into row B and mix by pipetting. Continue diluting to row G. This results in a twofold serial dilution from (16–0.25 μM).
Seal the plate with a gas-permeable membrane and incubate 18–20 h (see Note 4) at 37 °C and 220 rpm.
Remove the gas permeable membrane and measure OD600. The MIC is defined as the lowest concentration of MO with an average well OD600 within three standard deviations of the negative control wells (i.e.,—if the negative control is 0.048 ± 0.005, then the MIC would be wells with OD600 ≤ 0.063, see Fig. 1b).
Fig. 1.
Minimum Inhibitory (MIC) and Minimum Bactericidal Concentration (MBC) Assays. (a) MIC assay plate layout for testing six different morpholino oligomers (MOs) in duplicate from 16 μM to 0.25 μM. 200 μL of MO is added in duplicate to row A and diluted by serial twofold dilutions (100 μL transfer) to row G.Row H contains the positive and negative controls. (b) Mock OD600 sample data for a single MO. The dashed box indicates the MIC of 4 μM (lowest dilution with mean OD600 ≤ 0.063). (c) Mock CFU/mL data for rows A–C in (b). The inoculum concentration was 5.2 × 105 CFU/mL. The MBC is defined as a 3-log reduction (≤5.2 × 102 CFU/mL), so the MBC is 16 μM
3.2 Minimum Bactericidal Concentration (MBC) Assay
Conduct an MIC assay as in Subheading 3.1.
Serially dilute and plate all wells with no growth (OD600 ≤ 0.063 in this example, rows A–C in example Fig. 1b).
Incubate plates overnight at 37 °C.
Determine average CFU/mL for each MO concentration. The MBC is defined as a 99.9% (3-log) reduction in bacterial number from the starting inoculum step 5 of Subheading 3.1 (see Fig. 1c).
3.3 Synergy Assay
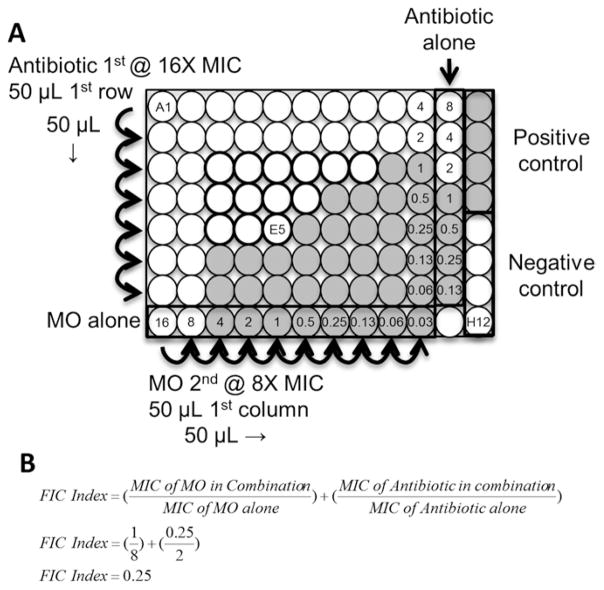
The synergy assay is basically two MIC assays performed perpendicularly on the same plate. Unlike the MIC assay, the bacteria are added after serial dilutions of the MO and antibiotic for ease of calculations. The MIC values for the MO and antibiotic of interest should be determined beforehand. The example volumes below are for one assay and mock MICs of 8 μM for the MO and 2 μg/ mL for the antibiotic (10 mg/mL stock). The antibiotic is diluted first and added at 16 times the MIC (32 μg/mL) and the MO is diluted second and added at 8 times the MIC (64 μM). This is done so that well A1 is twofold higher than the expected MIC of each agent. This assures the MIC is validated on the plate, as it is common for the MIC to be variable by twofold [1].
Add 50 μL of MHII to every well of a 96-well plate.
Prepare bacterial culture as in steps 1–3 of Subheading 3.1.
Add 120 μL of OD600 stock to 12 mL of MHII to attain 1 × 106 CFU/mL. Vortex vigorously and place on ice.
Serially dilute and plate to determine CFU/mL as in step 5 of Subheading 3.1 and Note 6.
Antibiotic will be diluted first, the desired final starting concentration is 4 μg/mL, which is twofold higher than the expected MIC of 2 μg/mL. Therefore a 16-fold concentration of the MIC is prepared (32 μg/mL). Add 2.4 μL of the 10 mg/ mL antibiotic stock to 747.6 μL of MHII. Vortex.
MO will be diluted second, the desired final starting concentration is 16 μM, which is twofold higher than the expected MIC of 8 μM. Therefore an eightfold concentration of the MIC is prepared (64 μM). Add 28.8 μL of the 1 mM MO stock to 421.2 μL of MHII. Vortex.
Add 50 μL of antibiotic stock to wells A1–A11 (see Fig. 2a).
Perform twofold dilutions by adding 50 μL of A1–11 to B1–11, mix. Repeat to row G1–11, skipping row H.
Add 50 μL of MO stock to wells of column 1 (see Fig. 2a).
Perform twofold dilutions by adding 50 μL to column 2, mix. Repeat to column 10, skipping columns 11 and 12.
Add 50 μL of 1 × 106 CFU/mL bacterial stock to all wells except E12–H12. Add 50 μL of MHII to E12–H12 (negative controls). See Fig. 2a and Note 9.
Seal the plate with a gas-permeable membrane and incubate 18–20 h (see Note 4) at 37 °C and 220 rpm.
Remove the gas permeable membrane and measure OD600 as in step 11 of Subheading 3.1.
Assess synergy by locating the lowest dilution combination well in which there is no growth (OD600 ≤ 0.063 in this example, see Fig. 2a, b). Calculate the fractional inhibitory concentration index (FIC index) using the equation in Fig. 2b (see Note 10):
Fig. 2.
Synergy Assays. (a) Synergy assay plate layout for testing a morpholino oligomer (MO) in combination with an antibiotic. 50 μL of antibiotic at 16 times the MIC is added to wells A1–11 and then serially diluted two-fold (50 μL transferred) to row G. 50 μL of MO at eight times the MIC is added to wells column 1 and then serially diluted twofold (50 μL transferred) to column 10. 50 μL of a 1 × 106 CFU/mL stock is added to all but the negative control wells which receive an additional 50 μL of MHII. After plating the bacteria is at 5 × 105 CFU/mL and well A1 contains MO at 16 μM and antibiotic at 4 μg/mL, while well G10 contains MO at 0.03125 μM and antibiotic at 0.0625 μg/mL. Note that the antibiotic only column is at twofold higher concentration than the other antibiotic-containing wells. Shaded wells represent mock growth wells. Dark outline wells indicate wells in which inhibition is below the MIC alone values. (b) Well E5 is the best interaction well. Demonstration of calculating the FIC Index for well E5
Footnotes
A spectrophotometer is not required and CLSI recommends visual comparisons using the unaided eye [1]. In the authors’ experience a 96-well plate reader is simply more efficient for reading multiple plates quickly and accurately.
Sterile untreated round-bottom 96-well plates provide the most optimal growth conditions. However, in the authors’ experience flat-bottom and tissue culture plates do not significantly alter MIC values. Due to the charge of tissue culture plates, novel cell-penetrating peptide-conjugated MOs should be compared in both plate types. This is due to the theoretical potential for charge-mediated interactions between MO and tissue culture treated plate.
The gas permeable membrane prevents evaporation in the wells. An 18 h incubation in a humid (water pan) incubator does not require the membrane, however it is recommended for longer incubations or when performed in nonhumid incubators.
These incubation times are for bacteria that reach late logarithmic or early stationary phase by 16–20 h. Incubation times should be increased for slower growing bacteria to the time required for a control culture to reach this growth stage.
As a note, the commercially available 0.5 McFarland standard may also be used as its optical density is also roughly equivalent to 1 × 108 CFU/mL.
The ideal inoculum is 5 × 105 CFU/mL and is based off the OD600 measurement in step 3 of Subheading 3.1. Each organism’s growth rate is different, so if the inoculum is not between 1 × 105 CFU/mL and 1 × 106 CFU/mL an adjustment is required. Divide the CFU/mL obtained in step 5 of Subheading 3.1 by the OD600 in step 3 of Subheading 3.1 to get CFU mL−1 OD−1. This number can then be used to estimate CFU/mL more accurately. e.g.,—The OD600 was 0.08 and yielded 3 × 106 CFU/mL. Step 4 of Subheading 3.1 is a 1:200 dilution, so the stock was 6 × 108 CFU/mL yielding 7.5 × 109 CFU mL−1 OD−1. For this specific organism the OD600 can be multiplied by 7.5 × 109 CFU mL−1 OD−1 to determine the CFU/mL. This value can be used to dilute to the desired 5 × 105 CFU/mL.
If there is no inhibition, increase the starting concentration of MO or antibiotic. However, do not add more than 10% of drug to the media (i.e.,—don’t add more than 45 μL of 1 mM to 405 μL of MHII). If required by volume constraint, increase the MO or antibiotic stock concentration utilized (e.g., 10 mM).
Antibiotics are added to row A in the synergy assay because they are generally more soluble and less costly than MO. However, the compounds can be loaded in alternate orientation. Remember that to reach the correct final concentration the first compound to be added should be prepared at eight times the desired starting concentration (2× the expected MIC in the example) and the second at four times the desired starting concentration. Also remember that the first compound to be diluted will be at 2× the concentration in the “alone” column (column 11 in the example).
Refer to the layout in Fig. 2a. After step 10 of Subheading 3.3, all wells contain 5 × 105 CFU/mL of bacteria, well A1 contains 16 μM MO and 4 μg/mL antibiotic while G10 contains 0.03125 μM MO and 0.0625 μg/mL antibiotic with every combination in the remaining wells. Column 11 and row H are antibiotic and MO only, respectively, to confirm MIC values. The antibiotic only column is at a twofold higher concentration due to one less dilution step (The MO dilution series skips this column). Column 12 contains positive (A12–D12) and negative (E12–H12) growth controls.
There are many interpretations of FIC indices in the literature [9]. We suggest a synergistic interaction when the FIC index is ≤0.5, an additive interaction when FIC index is between 0.5 and 1.0, and an antagonistic interaction when the FIC index is ≥1.0.
References
- 1.CLSI. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard-tenth edition. Clinical and Laboratory Standards Institute; Wayne, PA: 2015. [Google Scholar]
- 2.CLSI. Methods for determining bactericidal activity of antimicrobial agents; approved guideline. Clinical and Laboratory Standards Institute; Wayne, PA: 1999. [Google Scholar]
- 3.Moellering RC., Jr Rationale for use of antimicrobial combinations. Am J Med. 1983;75:4–8. doi: 10.1016/0002-9343(83)90088-8. [DOI] [PubMed] [Google Scholar]
- 4.Wu RP, Youngblood DS, Hassinger JN, Lovejoy CE, Nelson MH, Iversen PL, et al. Cell-penetrating peptides as transporters for morpholino oligomers: effects of amino acid composition on intracellular delivery and cytotoxicity. Nucleic Acids Res. 2007;35:5182–5191. doi: 10.1093/nar/gkm478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Good L, Awasthi SK, Dryselius R, Larsson O, Nielsen PE. Bactericidal antisense effects of peptide-PNA conjugates. Nat Biotechnol. 2001;19:360–364. doi: 10.1038/86753. [DOI] [PubMed] [Google Scholar]
- 6.Geller BL, Marshall-Batty K, Schnell FJ, McKnight MM, Iversen PL, Greenberg DE. Gene-silencing antisense oligomers inhibit acinetobacter growth in vitro and in vivo. J Infect Dis. 2013;208:1553–1560. doi: 10.1093/infdis/jit460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Greenberg DE, Marshall-Batty KR, Brinster LR, Zarember KA, Shaw PA, Mellbye BL, et al. Antisense phosphorodiamidate mor-pholino oligomers targeted to an essential gene inhibit Burkholderia cepacia complex. J Infect Dis. 2010;201:1822–1830. doi: 10.1086/652807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Howard JJ, Sturge CR, Daly SM, Gill M, Labandeira-Rey M, Harbour L, et al. ASM Microbe. Boston, MA: 2016. Inhibition of Pseudomonas aeruginosa by peptide-conjugated phosphorodiamidate morpholino oligomers. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elion GB, Singer S, Hitchings GH. Antagonists of nucleic acid derivatives. VIII. Synergism in combinations of biochemically related antimetabolites. J Biol Chem. 1954;208:477–488. [PubMed] [Google Scholar]