Figure 1. Comparison of pseudoenzyme scaffolds and AKAPs.
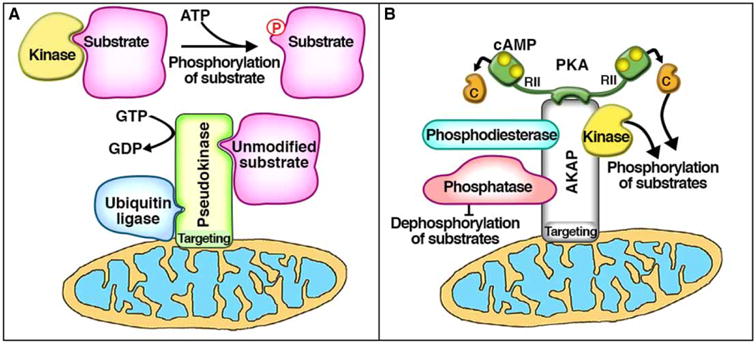
(A) Pseudoenzymes can act as an inhibitory anchor by binding and recruiting substrate protein away from active kinases and into a pseudoenzyme scaffold or ‘pseudoscaffold,’ thereby preventing substrate phosphorylation. This pseudoscaffold can possess additional features or functionalities such as (1) organelle-targeting motifs (i.e. mitochondrial, such as ADCK3), (2) separate catalytic sites (i.e. GTPase, such as MTMR5/13), or (3) other protein-protein interaction domains (i.e. heterodimerization, such as HER3; or other regulatory enzymes and their substrates, such as Trib2). Similarly, (B) AKAPs are a family of non-catalytic scaffolding proteins that, by definition, anchor the kinase PKA. In addition to PKA anchoring, AKAPs have also been shown to interact with a variety of other signaling molecules such as PDEs, phosphatases, and even other kinases. Many AKAPs also contain organelle-targeting motifs (i.e. mitochondrial, such as D-AKAP1). Through these additional features, both of these non-catalytic protein scaffolds can exhibit exquisite control of subcellular microdomains.
