Figure 6.
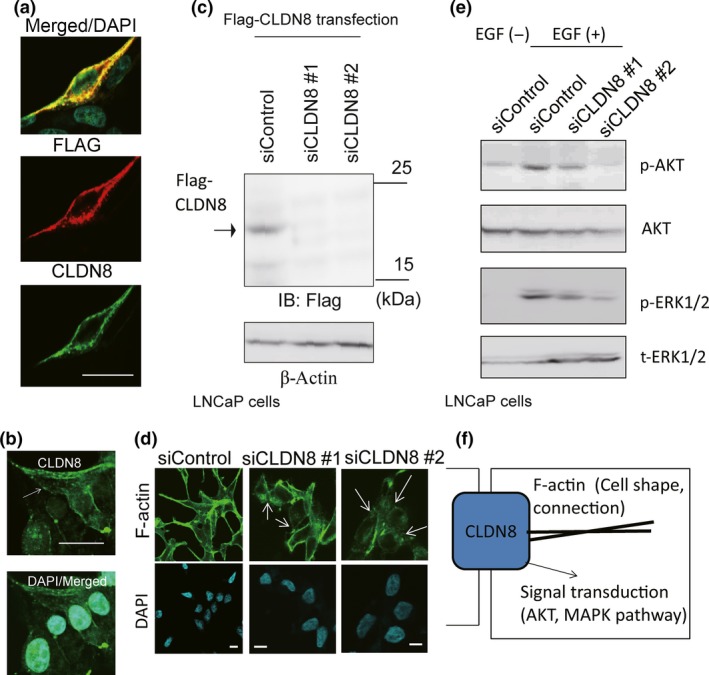
Knockdown of CLDN8 inhibited the stability of the cytoskeleton in LNCaP cells and collapsed tight junctions. (a) Overexpressed Flag‐CLDN8 was detected by anti‐CLDN8 antibody. Cells were transfected with Flag‐CLDN8 plasmid. After 48 h of incubation, immunocytochemical analysis was undertaken with anti‐Flag and anti‐CLDN8 antibody. Bar = 10 μm. (b) Analysis of localization of endogenous CLDN8 in LNCaP cells detected by anti‐CLDN8 antibody. Bar = 10 μm. (c) Verification of the effect of siRNAs targeting CLDN8 in LNCaP cells. Cells were transfected with Flag‐CLDN8 plasmid. Next day, control siRNA (siControl) or siRNAs targeting CLDN8 (siCLDN8 #1 and #2) were transfected. After 48 h, Western blot analysis was carried out to detect Flag‐CLDN8 expression by anti‐Flag antibody. IB, immunoblot. (d) Confocal microscopic changes in F‐actin cytoskeleton organization in LNCaP cells. Cells were treated with siControl or siCLDN8 #1 or #2 for 72 h. Arrows indicate the collapse of tight junctions and cell adhesions. Bar = 10 μm. (e) Knockdown of CLDN8 inhibits protein kinase B (Akt) and MAPK activation in LNCaP cells. Cells were treated with 10 ng/mL epidermal growth factor (EGF) for 10 min. Western blot analysis was undertaken to detect phosphorylated (p‐)Akt (Ser473), p‐ERK1/2, and total Akt and ERK1/2. (f) Summary of CLDN8 function in prostate cancer cells. CLDN8 is involved in cytoskeleton organization by regulating tight junction stability. In the cellular signal transduction, CLDN8 positively regulates Akt and MAPK phosphorylation pathways to promote cell proliferation or migration.
