Figure 1.
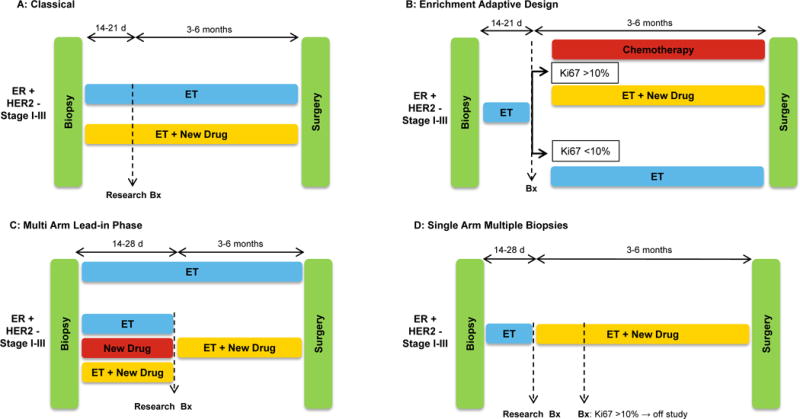
Different designs of NET trials (NET) trials in ER+/HER– breast cancer. A: Classical NET trial; patients receive the assigned therapy for the 3–6 months before surgery without any treatment modification; an on-treatment research biopsy is performed after 2–3 weeks. B: Enrichment Adaptive Design NET trial; patients receive endocrine therapy (ET) for 2–3 weeks and those patients whose tumors do not suppress Ki67 below a pre-established threshold are switched to chemotherapy or to the addition of a new drug, while those showing significant suppression of Ki67 continue on ET alone. C: Multi Arm Lead-in Phase NET trial; head to head comparison of ET± a new drug using the on-treatment 2-week Ki67 score as endpoint. Afterwards all patients receive ET + the investigational drug for 4–6 months before surgery. D: Single Arm Multiple Biopsies NET trial; patients receive ET for 2–4 weeks, time at which a research biopsy is performed and an investigational drug is added. After 2 additional weeks another biopsy is performed. Patients with cancers with a Ki67 ≥10% do not continue on study, while those with a Ki67<10% continue treatment with the combination until surgery.
