Fig. 3. TV-localized ML1 mediate Ca2+ release from TVs.
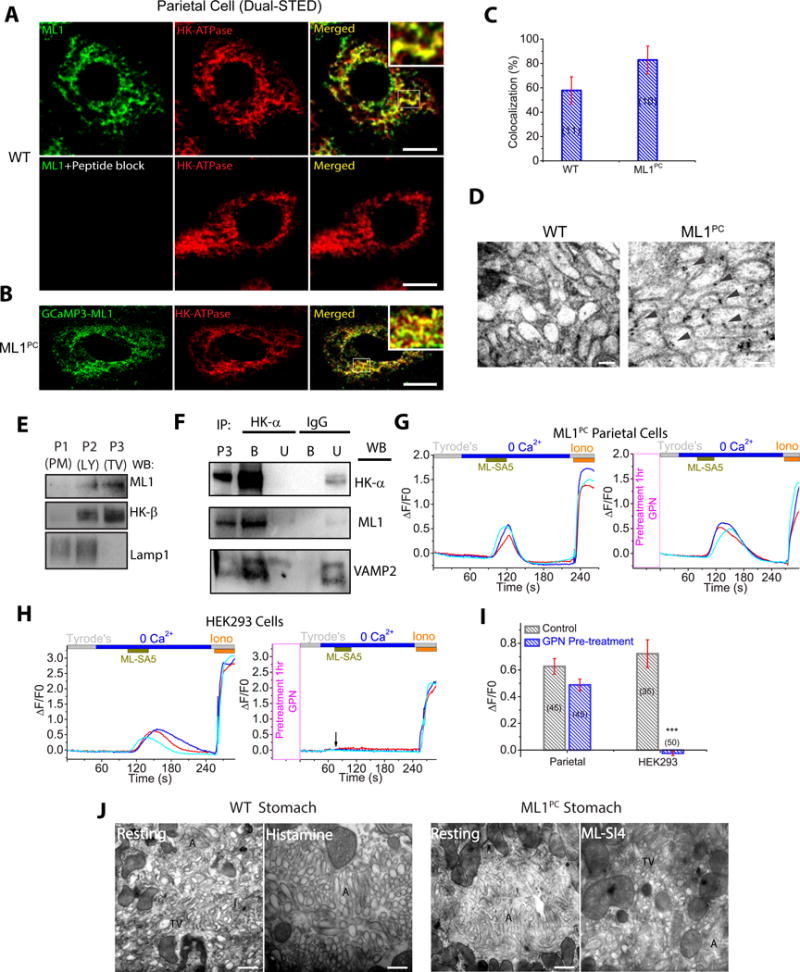
(A) Dual-STED images of WT corpus tissues immuno-labeled with anti-HK-α and anti-ML1. A pre-incubation of anti-ML1 with ML1 epitope peptide confirmed the specificity of the ML1 antibody. Scale bar, 5 μm. (B) Dual-STED images of ML1PC corpus tissues immuno-labeled with anti-HK-α and anti-GFP (recognizing GCaMP3-ML1). Note that constitutive TV exocytosis in ML1PC cells was blocked by ML-SI4 for this particular experiment. (C) Quantitative co-localization analysis of ML1 and H/K-ATPase based on randomly-selected images, as shown in A & B. (D) Immuno-gold electron microscopy images of WT and ML-SI4-treated (to prevent constitutive TV exocytosis) ML1PC parietal cells. Scale bar, 0.1 μm. (E) Gradient centrifugation purification of TVs from WT parietal cells. P1 (3,200 ×g), P2 (20,000 ×g), and P3 (100,000 ×g) pellets represent plasma membrane (PM)-rich, lysosome/mitochondria (LY), and H+/K+-ATPase-rich fractions (TV), respectively. (F) The expression of ML1, H+/K+-ATPase, and VAMP2 in TVs that were immunoisolated by anti-HK-α from P3 TV-derived membranes in E. IgG was used as a negative control. Western blot analyses were performed in both bound (B) immunoisolated vesicles and unbound (U) supernatants using P3 (see E) as a comparison. Shown were representative blots of three separate experiments. (G, H) Effects of GPN pretreatment on ML-SA5-induced Ca2+ release (GCaMP3 fluorescence, F480, in zero Ca2+ solution) from ML1PC parietal cells (G) or GCaMP3-ML1-transfected HEK293 cells (H). Cells were pretreated with ML-SI4 for 30 min to prevent TV exocytosis. (I) Quantitation of ML-SA5-induced GCaMP3 Ca2+ responses under control and GPN-pretreated conditions (mean ± SEM, n = 30–50 cells from ≤4 coverslips/experiment). ***P < 0.001 Student’s t-test. (J) TEM images of free TVs and apical canaliculi (A) in parietal cells. Scale bar, 0.5 μm.
