Table 2.
3-Hexyne Trimerization Dataa
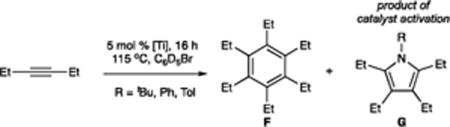
| ||||
|---|---|---|---|---|
|
| ||||
| [Ti] | % trimer yield | % [Ti] act.b | % conversion | TONc |
| 1 | 62 ± 13 | 80 ± 12 | 73 ± 17 | 4 ± 2 |
| 2 | 55 ± 21 | 57 ± 12 | 77 ± 12 | 5 ± 2 |
| 3d | 83 ± 6 | 17 ± 7 | 81 ± 3 | 35 ± 14 |
| 4 | 24 ± 8 | 33 ± 18 | 54 ± 16 | 5 ± 3 |
| 5 | 6 ± 5 | 42 ± 6 | 15 ± 0 | 1 ± 1 |
| 6 | 38 ± 2 | 48 ± 11 | 72 ± 18 | 5 ± 1 |
| 7 | 3 ± 3 | 70 ± 9 | 49 ± 11 | 0 ± 0 |
| 8 | 68 ± 7 | 74 ± 14 | 87 ± 2 | 6 ± 1 |
| 9 | 1 ± 0 | 8 ± 2 | 12 ± 0 | 1 ± 0 |
| 10 | 0 ± 0 | 1 ± 0 | 7 ± 2 | 0 ± 0 |
| 11 | 3 ± 0 | 41 ± 7 | 49 ± 8 | 1 ± 0 |
| 12 | 0 ± 0 | 7 ± 4 | 42 ± 13 | 0 ± 0 |
| 13 | 0 ± 0 | 0 ± 0 | 42 ± 11 | |
| 14 | 2 ± 2 | 6 ± 4 | 20 ± 15 | 2 ± 2 |
| 15 | 1 ± 0 | 25 ± 1 | 22 ± 6 | 0 ± 0 |
| 16 | 1 ± 0 | 0 ± 0 | 35 ± 4 | |
Conditions: 5 mol % [Ti], 0.4 M 3-hexyne, C6D5Br, 16 h, 115 °C, average of 2–4 runs. Quantitation determined by in situ 1H NMR.
% Ti activated = (yield of G)/[Ti]tot.
TON = (yield of F)/Ti activated.
Room temperature.
