Abstract
Background
Preoperative low-dose whole-body irradiation (IRR) with 1.5 and 7 Gy thymic IRR of the recipient, combined with a perioperative donor splenocyte infusion lead to reliable donor specific peripheral tolerance in our allogeneic porcine lung transplantation model. To reduce the toxicity of this preconditioning regime, modifications of the IRR protocol and their impact on allograft survival were assessed.
Methods
Left-sided single lung transplantation from major histocompatibility complex and sex mismatched donors was performed in 14 adult female minipigs. Recipient animals were exposed to 3 different protocols of nonmyeloablative IRR within 12 hours before transplantation. All animals were administered a donor splenocyte infusion on the day of lung transplantation. Intravenous pharmacologic immunosuppression was withdrawn after 28 postoperative days. Allograft survival was monitored by chest radiographs and bronchoscopy.
Results
IRR prolonged transplant survival in a dose- and field-dependent manner. Shielding of the bone marrow from IRR (total lymphoid IRR at 1.5 and 7 Gy thymic IRR) significantly reduced protocol toxicity defined as thrombocytopenia and consecutive increased bleeding propensity, but had a less effective impact on graft survival. Whole-body IRR at 0.5 and 7 Gy thymic IRR proved to be ineffective for reliable tolerance induction. Eventually, high levels of circulating CD4+CD25high regulatory T cells were present in long-term survivors.
Conclusions
These data show that the infusion of donor-specific alloantigen in combination with IRR is efficient once a threshold dose is exceeded.
The induction of tolerance toward a transplanted organ without the need for permanent immunosuppressive therapy is the goal in transplantation research.1,2 Continuous immunosuppression is associated with severe side effects, such as infections, neoplastic malignancies, and renal failure.3,4 An important part of tolerance induction is the preconditioning regime of the organ recipient. In our porcine allogeneic lung transplantation model, low-dose irradiation (IRR) was used to achieve a status of lymphocyte depletion, which is favorable for induction of graft tolerance and proved to generate allograft survival in a substantial proportion of animals.5 If this treatment was combined with a donor-specific or third-party splenocyte infusion (SpTx) on the day of transplantation, outcome was further improved, whereas SpTx alone failed to prolong allograft survival.6 Still, IRR in this manner is cause to serious physical adverse effects, such as nausea, decreased blood coagulation or anemia, and therefore lacks in clinical feasibility.7 Aiming to reduce these side effects, our established IRR regimen was further modulated in this study. Different IRR protocols where analyzed with respect to the resulting allograft survival and clinical applicability. We could show earlier that the augmentation of donor cell chimerism is positively correlated to a state of tolerance toward the lung allograft.6 As a prerequisite for the donor cells to be able to coexist, it was necessary to create space by depleting recipient cells. We hypothesized that herein a certain threshold of depletion must be exceeded. FACS analysis provided information about putative regulatory T (Treg) cells, namely CD4+CD25high T cells, which may be required to maintain allograft tolerance.8,9
MATERIALS AND METHODS
Animals
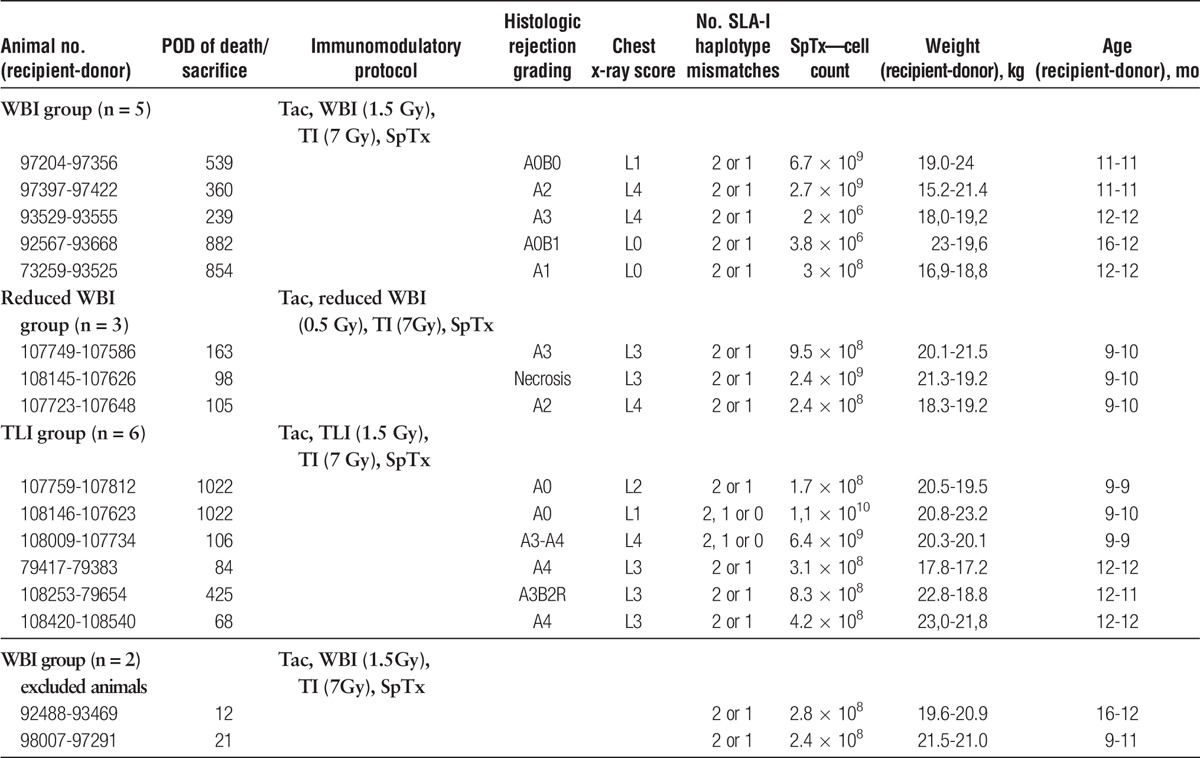
Fourteen Göttingen Minipigs (Table 1), originating from an outbred, specific-pathogen-free herd consisting of 9 different breeding lines, were obtained from Ellegaard (Dalmose, Denmark). They were tissue-typed prospectively by a lymphocytotoxic assay. Major histocompatibility complex (MHC) I mismatch was accomplished for haplotypes DC45 and W12 as well as haplotype d-specific monoclonal antibody 74-11-10. MHC II incompatibility was confirmed via reverse transcriptase PCR and subsequent sequencing of the swine leukocyte antigen (SLA) DQB gene. All animals received humane care in compliance with the German animal protection legislation, approved by the local Institutional Animal Care and Research Advisory Committee and permitted by the Animal Welfare Service of the Lower Saxony State office for Consumer Protection and Food Safety.
TABLE 1.
Animals
Surgical Technique
The surgical technique of left-sided single lung transplantation has been described in detail before.10 Briefly, lungs were harvested from male, porcine donors after Euro Collins cold flush perfusion. After thoracotomy in the 4th intercostal space the left lung was removed. The allogeneic lung was transplanted using a telescoping bronchial anastomosis technique with running posterior wall and interrupted anterior wall 4-0 polydioxanone sutures. The venous atrial cuff and the pulmonary artery were anastomosed with running polypropylene sutures.
Experimental Groups
Three different groups were assigned per the preconditioning regime intended for tolerance induction:
Whole-body IRR (WBI) with 1.5 Gy, plus thymic IRR (TI) with 7.0 Gy in a cervico-sternal field of 6 cm width × 12 cm length (Figure 1A), plus SpTx; WBI group, n = 5.
Reduced WBI with 0.5 and 7.0 Gy TI plus SpTx; reduced WBI group, n = 3.
WBI with 1.5 Gy was applied while shielding the bone marrow from radiation (Total lymphoid IRR, total lymphoid irradiation (TLI), Figure 1B) plus TI with 7.0 Gy plus SpTx; TLI group, n = 6.
FIGURE 1.
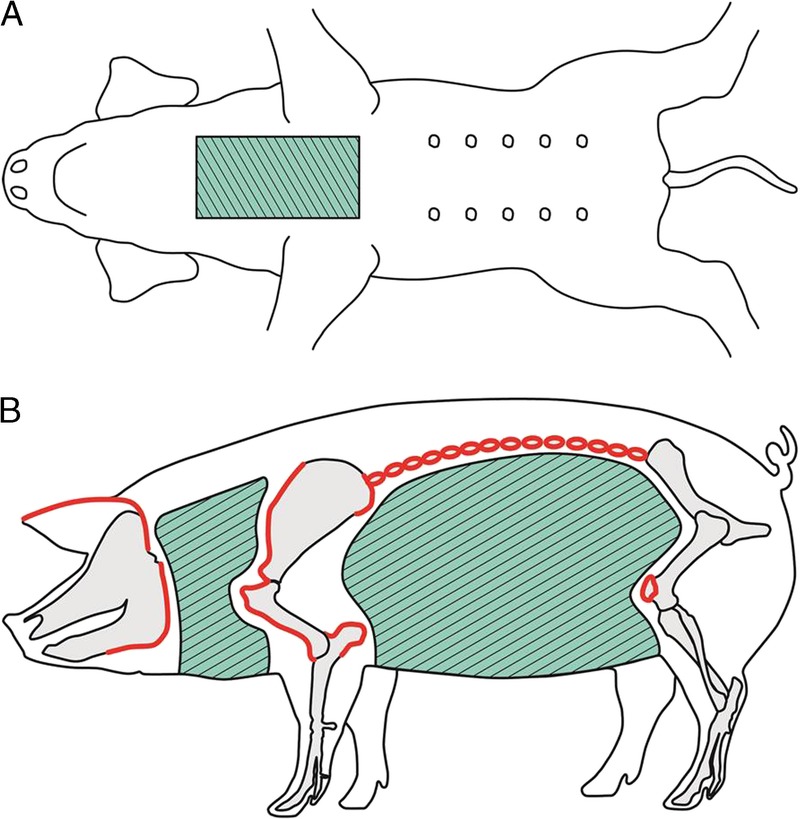
IRR fields. A, For thymic IRR (TI), a field (green crosshatched) of 6 cm width × 12 cm length, originating from the manubrium sterni, was exposed to a radiation dose of 7.0 Gy. B, For total lymphoid IRR (TLI) 1.5 Gy (0.75 Gy to each body side) were applied to a neck- and an abdominal field, to shield the bone marrow from radiation. Anatomical boarders were ear-basis, mandible and scapula/humerus for the neck field, and transverse processes, tuber olecrani, and patella for the abdominal field (IRR fields green crosshatched, delineated anatomical structures in red).
The experimental setup is shown in Figure 2. An overview of the animals and the respective protocol is listed in Table 1.
FIGURE 2.
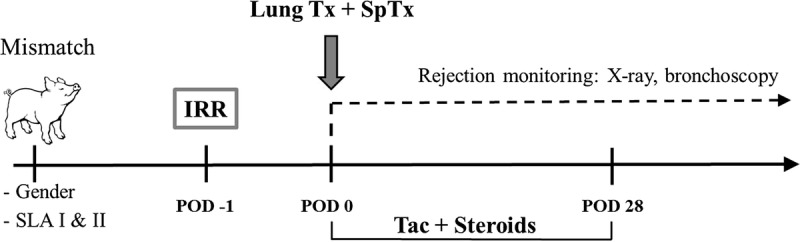
Experimental setup. Sex- and SLA-mismatched minipigs were selected before the start of the study. Recipient animals were irradiated (IRR) 1 day before transplantation in varying manners. On the day of transplantation (lung Tx) the pigs were administered a SpTx from their respective donor. After transplantation, animals were treated with Tacrolimus (Tac) and steroids for 28 days. Rejection was monitored by chest X-rays and bronchoscopies.
All IRR protocols were carried with a linear accelerator (Siemens and Elekta linear accelerators) 12 hours before transplantation. Immunosuppressive therapy consisted of Tacrolimus (Astellas, Tokyo, Japan) adjusted to 16 to 26 ng/mL blood trough levels and 1.5 mg/kg per day methylprednisolone. Two mg/kg per day Ciprofloxacin (Bayer, Leverkusen, Germany) was administered as intravenous antibiotic therapy. Immunosuppressives were withdrawn on postoperative day (POD) 28.
Preparation of Splenocytes
Spleens were disrupted mechanically, placed in phosphate-buffered saline and incubated with DNAse and collagenase (both Sigma, St. Louis, MO). The mixture was pushed through a mesh screen to retain residual splenic capsule and connective tissue. Splenocytes were isolated by density gradient centrifugation and afterwards filtered through a nylon mesh (100 μm) to remove cell aggregates. After washing, the suspension was administered intravenously after reperfusion of the transplanted lung. Characterization and composition of the SpTx has been described before.6
Rejection Monitoring
Chest radiographs and bronchoscopies were performed on POD 7, 21, 28, 42, 70, and 120. Afterward, they were continued in regular intervals per clinical condition. For the radiographs, a score from L0 (no pathological findings) to L4 (homogenous infiltration of the left lung and normal right lung) was assessed by a blinded reviewer.
In case clinical condition, chest radiograph and bronchoscopy indicated rejection of the graft, the animals were sacrificed and a full necropsy was performed. Histologic acute or chronic rejection was graded per the International Society for Heart and Lung Transplantation guidelines ranging from A0 to A4 and B0 to B4.11 Rejection was defined as a strictly left-sided infiltrate of the chest radiograph scored 2 or higher together with a grade A2-A4 or B3-B4 histologic rejection in the absence of infection. Only animals without signs of rejection at the time of immunosuppression withdrawal on POD 28 were included into the study.
Flow Cytometric Analysis
Peripheral blood monocytes were isolated by density gradient centrifugation from heparinised whole blood taken 1 hour after reperfusion, 1 hour after SpTx, on POD 1, 3, 5, 7, 14, 21, 28, 42, 70, 120, and subsequently together with the rejection controls. 2 × 105 cells were distributed to around bottom tubes and blocked with porcine serum. Staining was accomplished with CD4a primary antibody, harvested from hybridoma cell culture supernatant (clone 74-12-4; ATCC, Manassas, VA) and CD25 antibody (VMRD, Pullman, WA). For secondary staining of CD4a luorescein isothiocyanate-conjugated rat–anti-mouse IgG2b was used, for CD25 anti IgG1 PE (both BD Pharmingen, San Diego, CA) was used. Single cell staining with both secondary antibodies served as negative control. To exclude dead cells from analysis, propidium iodide (Sigma) was added to the samples. Flow cytometry was performed on a FACScan flow cytometer (BD, San Jose, CA). Data were analyzed using FACSDiva 6.0 software (BD).
Sequencing of SLA-DQB
Complete RNA was isolated from native lung tissue before transplantation from both, donor and recipient, with NucleoSpin RNA Kit (Macherey & Nagel, Dueren, Germany). Subsequent cDNA synthesis was accomplished with Omniscript Reverse Transkriptase-Kit (QIAGEN, Hilden, Germany) with oligo-dT Primers. cDNA was amplified with HotStar Taq polymerase (QIAGEN) using oligonucleotide primers ScDQB5a: 5′-GGC TGT GTT GAC TAC CAT TA-3′ and ScDQB3a: 5′-AGA CCA GCA GGT TGT GG-3′ for the second exon (β1 domain) of the MHC II molecule.12 Clean up of DNA was facilitated by NucleoSpin Extract II (Macherey & Nagel, Dueren, Germany). Sequencing of both strands was done by SeqLab (Goettingen, Germany).
Donor Leukocyte Chimerism
Chimerism was analyzed by quantitative PCR of the swine male-specific repeat (MSR) DNA present on the Y-chromosome.13 Briefly, blood DNA was extracted from isolated peripheral blood monocytes using the DNeasy Tissue Kit (Qiagen, Venlo, NL). Genomic DNA was amplified with MSR or control primers listed below. Cycling was performed on an iCycler (Biorad, Hercules, CA). SybrGreen (also Biorad) was used for signal induction. Data were analyzed using the iCycler software. Primers were MSR upper 5′-CCA TCG GCC ATT GTT TTC CTG TTC A-3′, MSR lower 5′-CCT CTG TGC CCA CCT GCT CTC TAC A-3′, S100C upper 5′-ATG CTG GAA GGG ACG GTA ACA ACA-3′ and S100C lower 5′-GCT CAG CTG CTG TCT TTC ACT CGT-3′.
Statistical Analysis
Statistical analysis was performed using GraphPad Prism 5.0 for Windows (GraphPad Software, San Diego, CA). For comparisons between the groups, the Mann-Whitney U test and for survival analyses the Gehan-Breslow-Wilcoxon test was used. P values less than 0.05 were considered significant. Results are presented as means ± SEM.
RESULTS
Effect on Differential Blood Cell Counts
In a porcine allogeneic lung transplantation model, different IRR protocols were administered 12 hours before surgery. Differential blood cell counts were used as indicators for potency of IRR. In all depicted parameters, cell counts in the WBI group were significantly lower than that in the 2 groups with modulated IRR protocols.
Lymphocyte depletion was most profound in the group receiving WBI. The lymphocyte course in the reduced WBI and TLI group was very like each other, only differing between POD 20 and 40, where in the TLI group, lymphocyte cell counts were falling again, whereas they increased in the reduced WBI group. After POD 42, a recovery in the lymphocyte population could only be seen in the reduced WBI group, whereas animals from the WBI group as well as from the TLI group did not reach their basal values until POD 100 (Figure 3A). Red blood cell counts were very much alike to the lymphocyte counts (Figure 3B).
FIGURE 3.
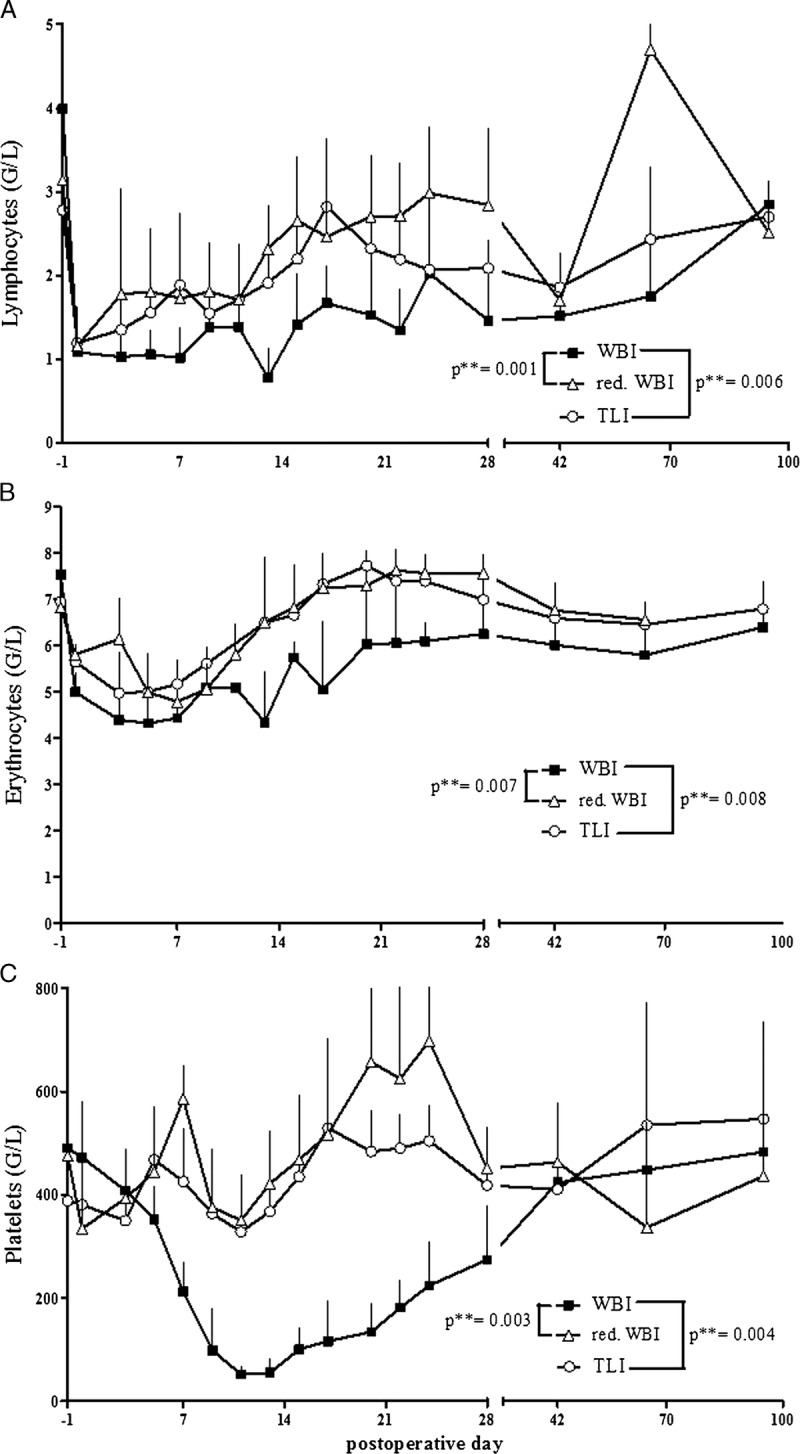
Differential blood cell counts in the respective groups. A, The weakest effect concerning lymphocyte depletion was observed in the reduced WBI group. B, Red blood cell counts were nearly identical in the reduced WBI and TLI groups, but significantly lower in the WBI group. C, WBI-treated animals showed a strong decrease of platelets during the period between POD 5 and 42, whereas in all other groups, the cell count remained stable.
WBI-treated animals showed a vigorous decrease of platelets during the period between POD 5 and 42, whereas in the other groups, platelet counts remained stable (Figure 3C).
Toxicity Related to Fashion and Dose of IRR
The huge deprivation of platelets observed in differential blood cell counts of the WBI group was accurately reflected in the physical condition of the animals. Two animals (recipients 92488 and 98007, Table 1) that originally belonged to this treatment group died due to spontaneous intrathoracic bleeding on POD 12 and 21, respectively, and therefore had to be excluded from further analysis. No such complications occurred in the 2 groups with modulated IRR protocols.
Allograft Survival
Reduced WBI, administered at 0.5 Gy WBI and 7.0 Gy TI along with a SpTx, clearly failed in the induction of tolerance. Rejection was detected in all animals after a median of 105 days. Compared with this group, survival in the WBI reference group was significantly higher (**P = 0.004). With this protocol, it was possible to induce tolerance in 60% of the recipient animals. With respect to the possible applicability in the clinical routine of human lung transplantation, the IRR regime was again altered in the TLI group, where the bone marrow of the animals was excluded from IRR. Although, after this treatment several rejections occurred during the first 100 POD, 33% of the animals developed stable tolerance toward their lung allografts, resulting in a median allograft survival of 265 POD (Figure 4).
FIGURE 4.
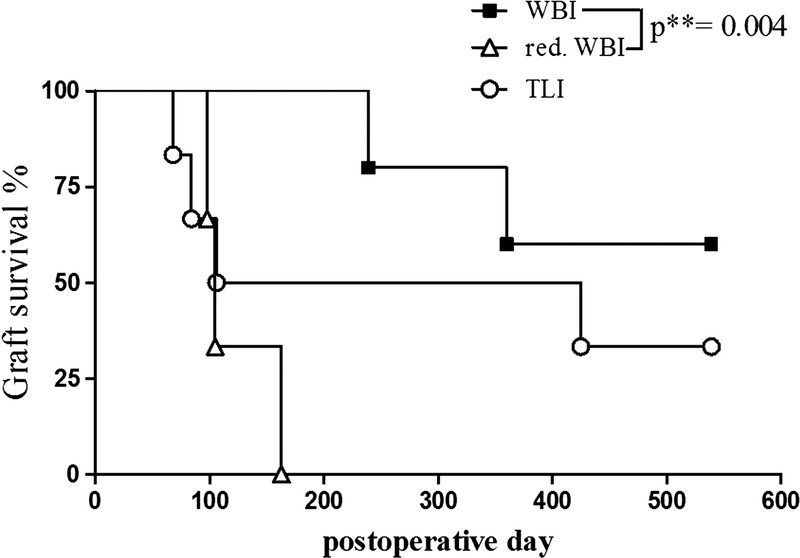
Kaplan-Meyer survival curve. Per the Gehan-Breslow-Wilcoxon test, survival differed significantly between the treatment groups WBI and reduced WBI (**P = 0.004). The TLI group with 3 early rejectors but also 2 long-term surviving animals showed no statistical significance to either side.
Development of CD4+CD25high Treg Cells
As described before, long-term survival of lung transplants is associated with an enhanced frequency of CD4+CD25high T cells.14,15 In this study, the frequency of CD4+CD25high T cells was compared with respect to the applied IRR protocol. After an initial increase in the WBI group 12 hours after radiation exposure, the percentile cell numbers temporarily decreased due to the depleting effect of the IRR regime with a nadir at POD 7. The 0.9% baseline was reached again at POD 28 in this group. After withdrawal of the pharmacological immunosuppression, putative Treg cell frequency increased. They even more than doubled their basal frequencies until POD 120.
The development of CD4+CD25high T cells in the TLI group followed a different pattern. Starting at 1.3%, IRR lead only to a little drop of cells on POD 0. From then on, they kept rising to a maximum of 2.5% on POD 42 but fell below baseline after that.
The relative decrease of CD4+CD25high T cells after IRR in the reduced WBI group compared to the starting point (2.2%) was even more profound than in the WBI group. Until POD 70, frequencies in the reduced WBI group were constantly higher than in the WBI group but dropped below basal levels on POD 120 (Figure 5A).
FIGURE 5.
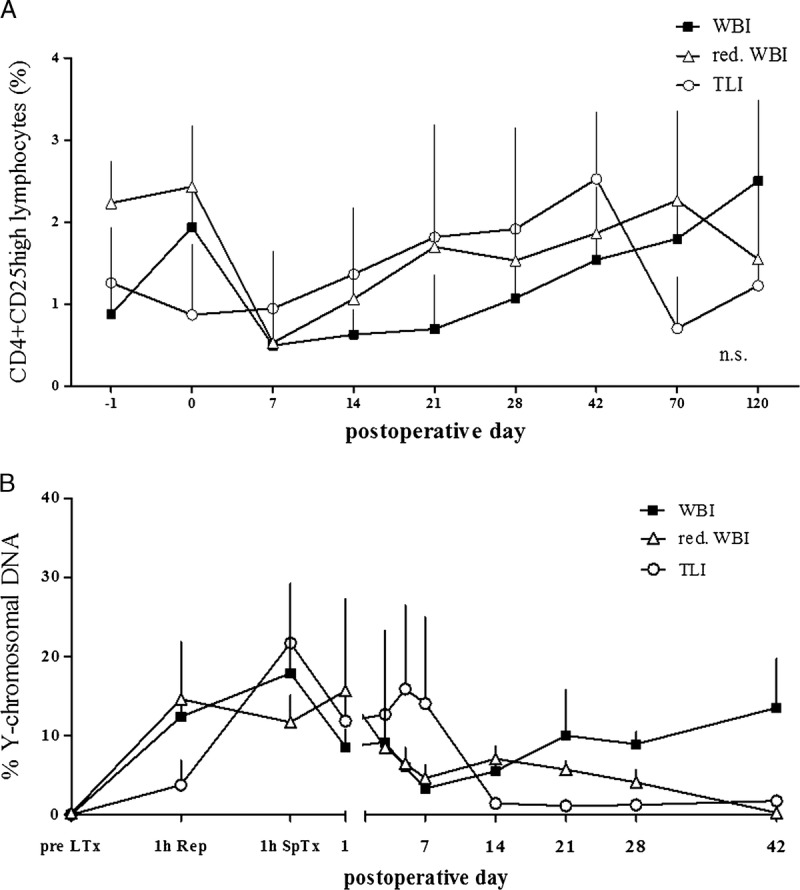
A, Development of CD4 + CD25 high Treg cell during 120 postoperative days. Due to completely different behaviour between the groups, there is no statistical difference, but the only constant ascending trend after withdrawal of immunosuppression can be seen in the WBI group. B, Donor cell chimerism in peripheral blood. The only group where donor cells seem to proliferate and a mixed chimerism is maintained is the WBI group.
Correlation of Peripheral Tolerance and Early Postoperative Chimerism
The first peak of donor leukocyte chimerism, as measured by RT Q-PCR for Y-chromosomal DNA in peripheral blood, was detected 1 hour after lung transplantation (up to 22%) in all groups. Thereafter, chimerism in the TLI group, as well as in the reduced WBI group was pretty much lost beyond POD 42. However, donor cells started to proliferate again in the WBI group after withdrawal of the immunosuppressive therapy on POD 28, becoming obvious in the once again increasing relative donor cell numbers (Figure 5B). Administrated splenocyte cell counts per kg body weight did not differ significantly between the groups (data not shown).
DISCUSSION
Here, we wished to study whether modifications of our established single low-dose IRR protocol (1.5 Gy WBI, 7.0 Gy TI) to induce allograft acceptance in an allogeneic lung transplantation model,6 would lead to comparable success about allograft survival. To reduce the toxicity of the IRR regimen and therefore improve the clinical applicability, 1 group was created that was exposed to only a 3rd of the original WBI (0.5 Gy) at unvarying TI (7 Gy). In another group, WBI was replaced by more selective TLI (1.5 Gy) at again unvarying TI (7 Gy). Ideally, on the one hand, preconditioning of the recipient animals should result in reliable T cell depletion, on the other hand, damage of other cell populations and the resulting side effects should be avoided. Differential blood cell counts revealed that in all parameters, cell depletion was significantly stronger in the WBI group than in the reduced WBI or TLI group (Figures 3A-C). This was a welcome effect in terms of preservation of all cell populations but lymphocytes. Especially sparing of the platelets and the consecutive reduced bleeding predisposition seemed a considerable improvement of the altered protocols, supported by the improved postoperative clinical course of these animals. Whereas 2 pigs that originally belonged to the WBI group died due to the deleterious consequences of thrombocytopenia within 3 weeks after IRR/transplantation, none of the modulated IRR protocols did lead to such undesired effects.
However, graft survival rates indicate that a certain degree of lymphocyte depletion is necessary for the prevention of allograft rejection (Figure 4). Compared with the WBI group with a survival of 60%, in the reduced WBI group, where lymphocyte depletion was least, all animals rejected their lung allografts within 163 days. Even though lymphocytes were only slightly more reduced in the TLI group, 2 of the 6 animals turned into long-term survivors. Consequently, there is reason to believe that a threshold dose of IRR is required, supported by Huang and colleagues16 who could show before that a WBI-dose of 1.0 Gy needed to be exceeded to allow bone marrow engraftment in their miniature swine model of skin transplantation. On the other hand, we assume that the localization of exposure can be restricted to the secondary lymphatic organs if the recipients' T cells are eliminated separately either by TI or administration of anti–T-lymphocyte antibodies. Until now, nonmyeloablative IRR in solid organ transplantation without permanent immunosuppression has successfully been established in preclinical small animal models.17,18 Specific unresponsiveness could be induced by preoperative TLI with 1.8 Gy and a 10-day course of ATG treatment after transplantation in a heterotopic heart transplantation study with mongrel dogs.19 As shown by several previous studies, allograft acceptance is positively correlated to the extent of space that could be created in the recipient animals, becoming obvious in lymphocyte cell counts and the amount of donor cell chimerism.5,6,20 These findings are in line with the results of our current study. Whereas all animals were administered a splenocyte cotransplantation on the day of transplantation, only the WBI group could maintain stable mixed chimerism beyond termination of the pharmacologic immunosuppression on POD 28 (Figure 5B). In transplantation of hematopoietic stem cells, TLI is already used, either in preclinical large animal models or as preconditioning regime in patients with malignancies,21 considering that the development of graft-versus-host disease (GvHD) is still a risk factor.22 However, protocols that prevent GvHD in stem cell transplantation are available.23,24 Furthermore, GvHD is absent when diverse IRR regimes in combination and donor-derived antigen presenting cells are applied in an experimental model of porcine lung transplantation.25
Corresponding to the lesser depletion of lymphocytes, frequencies of CD4+CD25high putative Treg cells were higher in the reduced WBI and TLI groups than in the WBI group (Figure 5A). Only in the late follow-up on POD 120, a distinct separation could be seen in terms of rising frequencies in the WBI reference group and falling frequencies in the reduced WBI group, indicating that high frequencies of putative Treg cells are necessary to maintain allograft acceptance in the long term but not crucial during the period of immunosuppression either by pharmaceuticals or IRR. Matching the survival rates, frequencies started to rise again also in the TLI group on POD 120. In contrast, it has been observed in human lung transplantation that donor-reactive Treg cell frequencies increased during acute rejection episodes, indicating that rising Treg cell frequencies alone were insufficient to prevent acute rejection.26
In renal transplantation, it became possible to induce tolerance with a nonmyoablative preparative regimen in nonhuman primates.27 These results were transferred into human kidney transplantation. Thymic IRR, a monoclonal CD2 antibody and a cytostatic agent, together with donor-bone marrow cotransplantation at the day of kidney transplantation led to sustainable tolerance in 4 of 10 HLA mismatched patients with end-stage renal disease, in 3 patients, it was at least possible to discontinue immunosuppression for up to 4 years.28,29
However, the induction of tolerance in allogeneic lung transplantation seems more difficult to achieve. A protocol consisting of WBI and TI, bone marrow Tx and monoclonal antibody treatment that had successfully been applied in kidney transplantation, failed to achieve long term graft survival in lung transplantation in cynomolgus monkeys.27 Only recently, Tonsho et al30 were the first to succeed in inducing tolerance to lung allografts in their nonhuman primate transplantation model. This highly complex protocol contained WBI as well as TI in the same dosages that we proclaimed as clinically untransferable, but in a delayed manner (4 months after transplantation) and might therefore be a prospective option nevertheless. Even though it has been shown that a brief postoperative course of high-dose tacrolimus in pigs led to long-term lung allograft survival in all treated animals,31 the side effects of tacrolimus target levels of up to 50 ng/mL in humen limit the clinical potential of this approach.
A recent study by Duran-Struuck et al32 gives a hint that the administration of ex vivo expanded recipient Treg cells promotes prolonged and high levels of multilineage allogeneic chimerism. Because these findings are in line with our understanding of a positive correlation between Treg cell, chimerism and prolonged allograft survival20 and the first patients treated with ex vivo–generated Treg cells included in the ONE-study did prove the safety of this cellular therapy,33 a consequent next step in our project should be the combination of the herein described TLI-protocol with the administration of ex vivo generated Treg cells.
Limitations of the Model
No SLA-DQB differences were detected within donor/recipient pair nos. 107586/107749 and 93555/93529. Both recipients rejected their allograft on POD 163 and POD 239, respectively. Above that, Sahara et al34 could show before that SLA-II molecules seem to be less antigenetic than SLA-I molecules. All other donor/recipient pairs showed differences in their SLA-DQB gene ranging from 1 up to 30 basepairs, regardless of the outcome.
Besides the increased expenses, the high SLA variability could be considered as a disadvantage in this porcine model, using outbred breeding lines. However, we regard this condition even as a strength of our model as, compared with artificially created inbred models, it reflects the concrete clinical situation. Experience shows that absent immunomodulatory manipulation leads to early postoperative allograft rejection.
CONCLUSIONS
The induction of long-term tolerance is a balancing act between sufficient overall cell depletion and the preservation of some immunity against environmental pathogens as well as an initial population of naturally occurring and developing Treg cell. Cell depletion was shown to be necessary to create space for donor cells for the induction of chimerism, whereas CD4+CD25high Treg cells facilitate long-term tolerance in this model of porcine allogeneic lung transplantation. Even though the most successful induction treatment in this study remained the WBI reference protocol, there could be found some promising aspects in the TLI group. Considering the good compatibility of our applied TLI protocol, a raise of dosage for the TLI IRR fields, which would result in higher lymphocyte depletion or a combination with T cell–depleting antibodies and/or ex vivo generated Treg cells, would be conceivable for future experiments.
ACKNOWLEDGMENTS
The authors thank Birte Kristensen for performing the SLA-typing and Karin Peschel, Astrid Diers-Ketterkat, and Petra Ziehme for their invaluable technical assistance. This study was supported by grants from the Biomedical Research in Endstage and Obstructive Lung Disease Hannover (BREATH), Member of the German Center for Lung Research (DZL), Hannover, Germany. Astellas, Osaka, Japan, kindly donated Tacrolimus.
Footnotes
Published online 6 June 2017.
K.J. and K.D. contributed equally to this work.
This study was supported by grants from the Deutsche Forschungsgemeinschaft (KFO 123, SFB738 project B3), as well as from the Biomedical Research in Endstage and Obstructive Lung Disease Hannover (BREATH), Member of the German Center for Lung Research (DZL), Hannover, Germany. The German Ministry for Education and Research BMBF 01EO1302. Astellas, Osaka, Japan, kindly donated Tacrolimus and supplied the project with a research grant.
The authors declare no conflicts of interest.
K.J. and K.D. participated in performance of the research, in data analysis and in writing of the article. W.S., M.A., J.S., and T.S. participated in performance of the surgical procedures. A.-K.K. and L.P. participated in data analysis. J.G., J.F., and M.W. contributed analytic tools. D.J. participated in data analysis. M.S., A.H., and G.W. participated in research design, performance of the research and data analysis.
REFERENCES
- 1.Floreth T, Bhorade SM. Current trends in immunosuppression for lung transplantation. Semin Respir Crit Care Med. 2010;31:172–178. [DOI] [PubMed] [Google Scholar]
- 2.Grinyo JM. Why is organ transplantation clinically important? Cold Spring Harb Perspect Med. 2013;3:a014985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scheffert JL, Raza K. Immunosuppression in lung transplantation. J Thorac Dis. 2014;6:1039–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nair N, Gongora E, Mehra MR. Long-term immunosuppression and malignancy in thoracic transplantation: where is the balance? J Heart Lung Transplant. 2014;33:461–467. [DOI] [PubMed] [Google Scholar]
- 5.Warnecke G, Avsar M, Morancho M, et al. Preoperative low-dose irradiation promotes long-term allograft acceptance and induces regulatory T cells in a porcine model of pulmonary transplantation. Transplantation. 2006;82:93–101. [DOI] [PubMed] [Google Scholar]
- 6.Avsar M, Jansson K, Sommer W, et al. Augmentation of transient donor cell chimerism and alloantigen-specific regulation of lung transplants in miniature swine. Am J Transplant. 2016;16:1371–1382. [DOI] [PubMed] [Google Scholar]
- 7.Scialdone L. Overview of supportive care in patients receiving chemotherapy: antiemetics, pain management, anemia, and neutropenia. J Pharm Pract. 2012;25:209–221. [DOI] [PubMed] [Google Scholar]
- 8.Kingsley CI, Karim M, Bushell AR, et al. CD25 + CD4+ regulatory T cells prevent graft rejection: CTLA-4- and IL-10-dependent immunoregulation of alloresponses. J Immunol. 2002;168:1080–1086. [DOI] [PubMed] [Google Scholar]
- 9.Hilbrands R, Howie D, Cobbold S, et al. Regulatory T cells and transplantation tolerance. Immunotherapy. 2013;5:717–731. [DOI] [PubMed] [Google Scholar]
- 10.Struber M, Hohlfeld JM, Kofidis T, et al. Surfactant function in lung transplantation after 24 hours of ischemia: advantage of retrograde flush perfusion for preservation. J Thorac Cardiovasc Surg. 2002;123:98–103. [DOI] [PubMed] [Google Scholar]
- 11.Yusen RD, Edwards LB, Kucheryavaya AY, et al. The registry of the International Society for Heart and Lung Transplantation: thirty-first adult lung and heart-lung transplant report—2014; focus theme: retransplantation. J Heart Lung Transplant. 2014;33:1009–1024. [DOI] [PubMed] [Google Scholar]
- 12.Shia YC, Bradshaw M, Rutherford MS, et al. Polymerase chain reaction based genotyping for characterization of SLA-DQB and SLA-DRB alleles in domestic pigs. Anim Genet. 1995;26:91–100. [DOI] [PubMed] [Google Scholar]
- 13.Gruessner RW, Levay-Young BK, Nakhleh RE, et al. Portal donor-specific blood transfusion and mycophenolate mofetil allow steroid avoidance and tacrolimus dose reduction with sustained levels of chimerism in a pig model of intestinal transplantation. Transplantation. 2004;77:1500–1506. [DOI] [PubMed] [Google Scholar]
- 14.Neujahr DC, Larsen CP. Regulatory T cells in lung transplantation—an emerging concept. Semin Immunopathol. 2011;33:117–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cobbold SP, Waldmann H. Regulatory cells and transplantation tolerance. Cold Spring Harb Perspect Med. 2013;3:a015545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang CA, Fuchimoto Y, Scheier-Dolberg R, et al. Stable mixed chimerism and tolerance using a nonmyeloablative preparative regimen in a large-animal model. J Clin Invest. 2000;105:173–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sharabi Y, Sachs DH. Mixed chimerism and permanent specific transplantation tolerance induced by a nonlethal preparative regimen. J Exp Med. 1989;169:493–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dodd-O JM, Ganguly S, Vulic A, et al. Induction of major histocompatibility complex-mismatched mouse lung allograft acceptance with combined donor bone marrow: lung transplant using a 12-hour nonmyeloablative conditioning regimen. Transplantation. 2016;100:e140–e146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Strober S, Modry DL, Hoppe RT, et al. Induction of specific unresponsiveness to heart allografts in mongrel dogs treated with total lymphoid irradiation and antithymocyte globulin. J Immunol. 1984;132:1013–1018. [PubMed] [Google Scholar]
- 20.Kruse B, Thissen S, Warnecke G, et al. Correlation of donor leukocyte chimerism with pulmonary allograft survival after immunosuppressive drug withdrawal in a porcine model. Transplantation. 2009;87:1468–1477. [DOI] [PubMed] [Google Scholar]
- 21.van Besien K. Allogeneic stem cell transplantation in follicular lymphoma: recent progress and controversy. Hematology Am Soc Hematol Educ Program. 2009:610–618. [DOI] [PubMed] [Google Scholar]
- 22.Kohrt HE, Turnbull BB, Heydari K, et al. TLI and ATG conditioning with low risk of graft-versus-host disease retains antitumor reactions after allogeneic hematopoietic cell transplantation from related and unrelated donors. Blood. 2009;114:1099–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sykes M. Mechanisms of transplantation tolerance in animals and humans. Transplantation. 2009;87(9 Suppl):S67–S69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johnston HF, Xu Y, Racine JJ, et al. Administration of anti-CD20 mAb is highly effective in preventing but ineffective in treating chronic GVHD while preserving strong GVL effects. Biol Blood Marrow Transplant. 2014;20:1089–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Warnecke G, Hutchinson JA, Riquelme P, et al. Postoperative intravenous infusion of donor-derived transplant acceptance-inducing cells as an adjunct immunosuppressive therapy in a porcine pulmonary allograft model. Transpl Int. 2009;22:332–341. [DOI] [PubMed] [Google Scholar]
- 26.Greenland JR, Wong CM, Ahuja R, et al. Donor-reactive regulatory T cell frequency increases during acute cellular rejection of lung allografts. Transplantation. 2016;100:2090–2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aoyama A, Ng CY, Millington TM, et al. Comparison of lung and kidney allografts in induction of tolerance by a mixed-chimerism approach in cynomolgus monkeys. Transplant Proc. 2009;41:429–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kawai T, Cosimi AB, Spitzer TR, et al. HLA-mismatched renal transplantation without maintenance immunosuppression. N Engl J Med. 2008;358:353–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kawai T, Sachs DH, Sprangers B, et al. Long-term results in recipients of combined HLA-mismatched kidney and bone marrow transplantation without maintenance immunosuppression. Am J Transplant. 2014;14:1599–1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tonsho M, Lee S, Aoyama A, et al. Tolerance of lung allografts achieved in nonhuman primates via mixed hematopoietic chimerism. Am J Transplant. 2015;15:2231–2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shoji T, Muniappan A, Guenther DA, et al. Long-term acceptance of porcine pulmonary allografts without chronic rejection. Transplant Proc. 2005;37:72–74. [DOI] [PubMed] [Google Scholar]
- 32.Duran-Struuck R, Sondermeijer HP, Buhler L, et al. Effect of ex vivo expanded recipient regulatory T cells on hematopoietic chimerism and kidney allograft tolerance across MHC barriers in cynomolgus macaques. Transplantation. 2017;101:274–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Edozie FC, Nova-Lamperti EA, Povoleri GA, et al. Regulatory T-cell therapy in the induction of transplant tolerance: the issue of subpopulations. Transplantation. 2014;98:370–379. [DOI] [PubMed] [Google Scholar]
- 34.Sahara H, Shoji T, Ng CY, et al. The role of indirect recognition of MHC class I and II allopeptides in a fully mismatched miniature swine model of lung transplantation. Transplant Proc. 2006;38:3256–3258. [DOI] [PMC free article] [PubMed] [Google Scholar]


