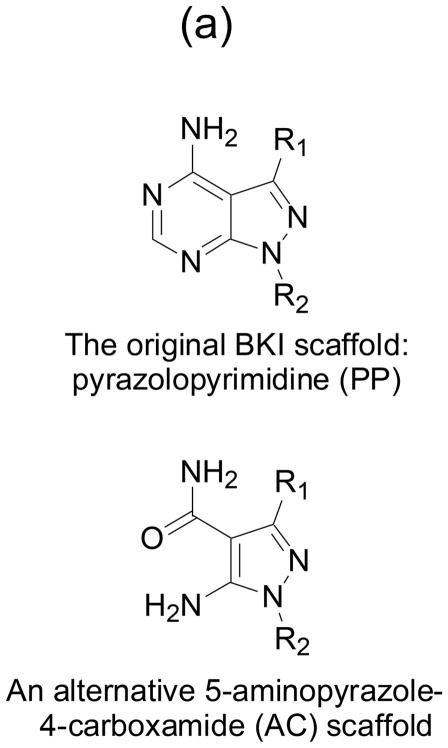
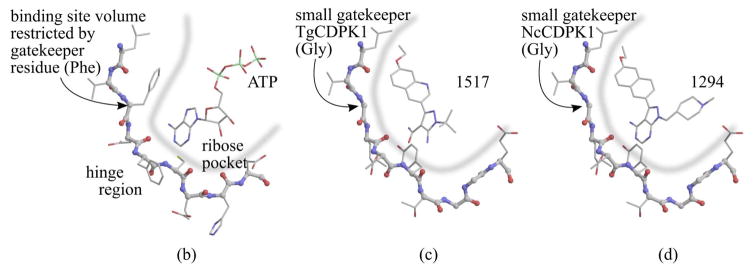
Fig. 1. BKIs core scaffold and orientation with enzymes ATP binding sites.
(a) Pyrazolopyrimidines (PP) and alternative 5-aminopyrazole-4-carboxamide (AC) scaffold chemical backbone for CDPK inhibitors. (b) Typical protein kinase active site. The volume accessible for ATP binding, indicated by the thick blue line, is limited by the presence of a large gatekeeper residue, in this case phenylalanine 103 of human CDK9. (c) Active site of T. gondii CDPK1 with AC scaffold BKI-1517. The large R1 substituent occupies a hydrophobic region made accessible by the absence of sidechain atoms in the glycine gatekeeper residue. (d) Active site of N. caninum CDPK1 with PP scaffold BKI-1294. In addition to the large R1 group, this inhibitor contains a large R2 group that extends deeper into the ribose pocket. The three crystal structures shown are 3BLQ, 4ONA, and 4MX9.