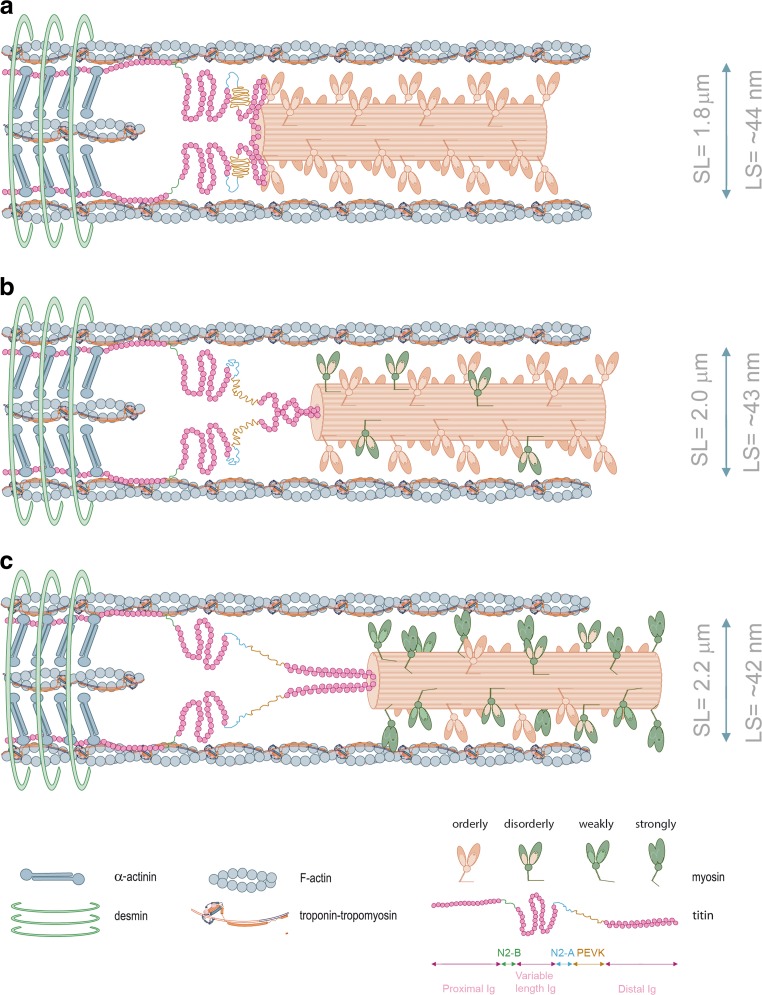
Fig. 1.
A schematic model of half-sarcomere at varying sarcomere lengths at low Ca2+ conditions. Lattice spacing dimensions at each varying length were taken from Konhilas et al. (2002a). As the muscle is stretched from a relatively short sarcomere length (a) to higher sarcomere lengths (b, c), lattice spacing becomes smaller, myosin approximates to actin and cross-bridges transit from orderly into disorderly states. The I-band region of titin is the extensible region and consists of three elastic components that act as a spring element: (1) tandem immunoglobulin (Ig)-like domain regions, with proximal (near Z-disc) and distal (near I-A regions) segments; (2) the PEVK sequence-region rich in proline (P), glutamic acid (E), valine (V) and lysine (K); and (3) the N2B and N2BA elements (both isoforms contain N2B segments, but only the N2BA isoform contains an additional N2A element) (Labeit and Kolmerer 1995). Titin-induced stretch imposes a passive strain on the thick-filament proteins, which reduces lattice spacing and changes the arrangement of cross-bridges. Upon fiber lengthening, titin anchorage both on actin and tropomyosin at the I-band region, imposes a passive strain on thin-filament proteins and increases the cooperative unit size. Please note that more myosin motors are turned “On” during systolic activation (Reconditi et al. 2017). Distinct myosin colors are depicted to better illustrate the transition of orderly to disorderly projections. α-actinin and desmin illustrate the Z-disc border. Note: cardiac myosin-binding protein C (cMyBP-C) was omitted to simplify the drawing and the width and sarcomere length dimensions are not to scale. (image adapted from Sequeira and van der Velden 2015)