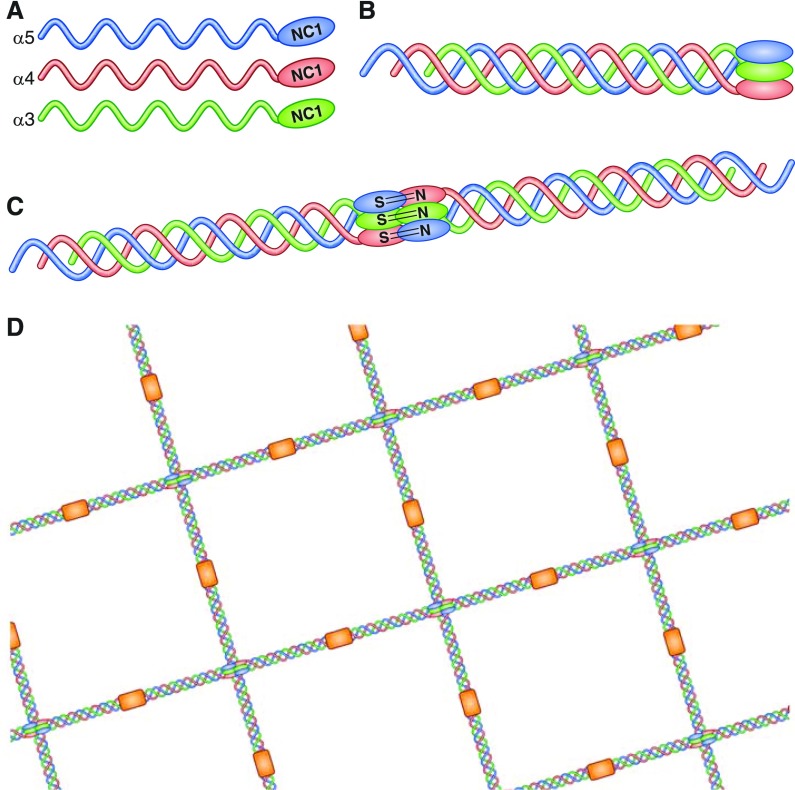
Figure 1.
Structure of the glomerular basement membrane. In its native form, the collagen IV network in the glomerular basement membrane consists of triple-helical protomers of α3, α4, and α5 chains (shown individually in [A]). The carboxy-terminal domains of these α3 α4 α5 protomers form a trimeric “cap” (B), end-to-end association of which results in the formation of the hexameric NC1 domain (C). The quaternary structure of this hexamer is stabilized by hydrophobic and hydrophilic interactions across the planar surfaces of opposing trimers, and reinforced by sulfilimine bonds crosslinking opposing NC1 domains. Two key autoantibody epitopes within α3(IV)NC1 have been described, designated EA (incorporating residues 17–31 toward the amino terminus) and EB (residues 127–141 toward the carboxy terminus), which in the native form are sequestered at the junction with α4 and α5 chains within the triple helical structure. Binding through 7s domains (shown in orange) completes the lattice-like structure of the type IV collagen network (D). Reprinted from reference 102.