Figure 1.
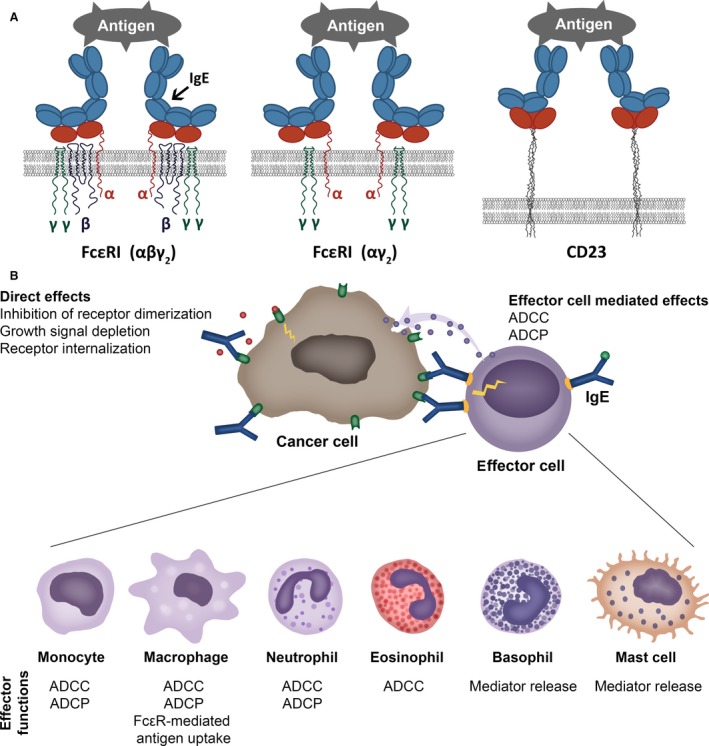
Cell surface IgE receptors and IgE‐mediated direct and indirect effects. (A) Cartoon of IgE binding to its cell surface receptors. IgE binds to tetrameric (αβγ2) (left) and trimeric forms (αγ2) (middle) of FcεRI through the extracellular domain of the alpha (α) chain of the receptor. The low‐affinity receptor CD23 trimer binds IgE through recognition of the lectin domain (right). (B) Direct and cell‐mediated effects of antitumour IgE. Like IgG antibody therapies, IgE targeting tumour antigens can exert direct effects through recognizing the target antigen, such as interference with signalling, resulting in growth inhibition. IgE can also bind via IgE receptors (FcεRI or FcεRII/CD23) to a specific repertoire of effector cells (illustrated in the bottom panel). These interactions may lead to effector functions against tumour cells, such as antibody‐dependent cell‐mediated phagocytosis (ADCP) or cytotoxicity (ADCC), or mediator release. Cross‐linking of IgE is required for effector cell activation, whereas soluble tumour antigens expressing only a single epitope do not trigger IgE cross‐linking on the surface of effector cells.
