Fig. 1.
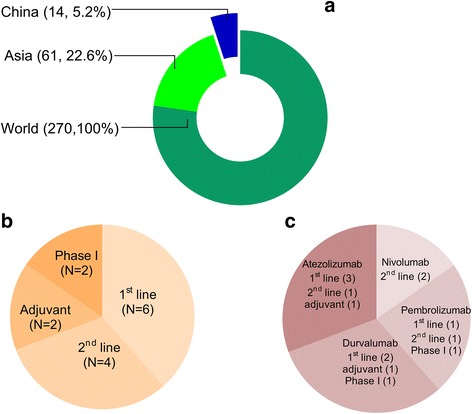
Ongoing international clinical trials according to whether they included Chinese patients. a Between January 1, 2013, and April 6, 2017, there were 270 clinical trials of anti-PD-1/PD-L1 inhibitors for NSCLC that were registered on ClinicalTrials.gov. Among the 270 studies, 61 studies were performed in East Asia and 14 studies were performed in China (12 multinational trials and 2 trials that only evaluated Chinese patients). b The 14 clinical trials included six first-line studies, four second-line studies, two adjuvant therapy studies, and two phase I studies for only Chinese patients. c The classification of clinical trials in China according to the therapeutic agent, which includes nivolumab, pembrolizumab, atezolizumab, and durvalumab
