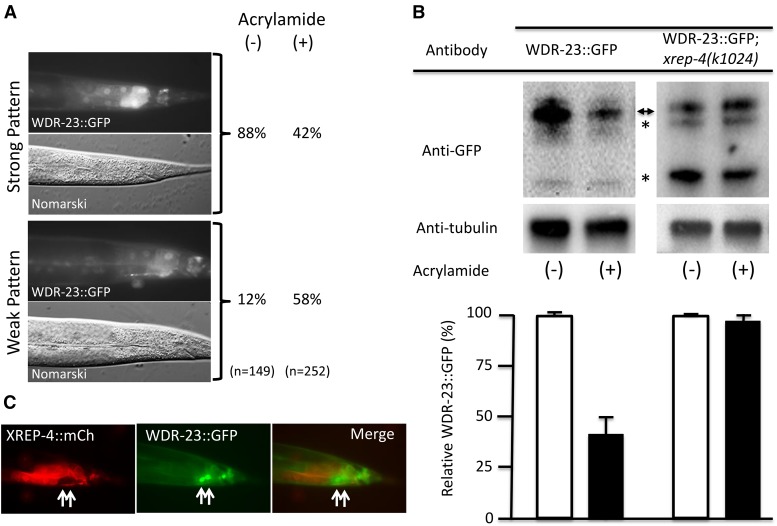
Figure 6.
WDR-23 levels are dynamic and dependent on stress and wild-type XREP-4 activity. (A) WDR-23::GFP levels are reduced after acrylamide exposure. Adult animals were scored for WDR-23::GFP signal intensity in posterior intestinal cells after culturing for ∼24 hr in the absence (−) or presence (+) of acrylamide. GFP levels were binned after scoring as either strong (upper panels) or weak (lower panels), revealing that the fraction of animals with strong expression was dramatically reduced (88–42%) after acrylamide exposure. (B) Total WDR-23::GFP protein levels are reduced after acrylamide exposure by an xrep-4-dependent mechanism. Western blots of total protein isolated from L3-L4 stage populations harboring an integrated wdr-23::gfp translational fusion transgene in either a wild-type (left panels) or xrep-4(k1024) mutant background (right panels); these L3–L4 animals had been cultured for ∼24 hr in the absence (−) or presence (+) of acrylamide. After probing with antibodies to detect GFP and the control protein tubulin, all band intensities corresponding to full length WDR-23::GFP (double arrowhead) and presumed degradation products (asterisks) were quantified, normalized to tubulin, and plotted below the corresponding lanes. Marked decreases in the relative GFP levels were detected after acrylamide exposure in the wild-type background. In contrast, WDR-23::GFP levels did not change at all in the xrep-4(k1024) mutant background, although we did note a change in the relative GFP-positive band intensities compared to the wild-type background. (C) XREP-4::mCh and WDR-23::GFP reporter patterns are mutually exclusive. Double transgenic adult animals harboring an extrachromosomal xrep-4::mCh and integrated wdr-23::gfp functional, translational transgene were assayed for reporter gene expression. Intestinal cells with low levels of XREP-4::mCh had strong WDR-23::GFP signals (arrows). Thus, the expression of xrep-4::mCh alone was sufficient to downregulate WDR-23::GFP, even in the absence of acrylamide exposure.