Figure 6. Conserved and species-specific editing sites affect protein function.
Unedited (wt) and singly-edited versions of the voltage-dependent K+ channels of the Kv2 subfamily were studied under voltage-clamp (see Table S9).
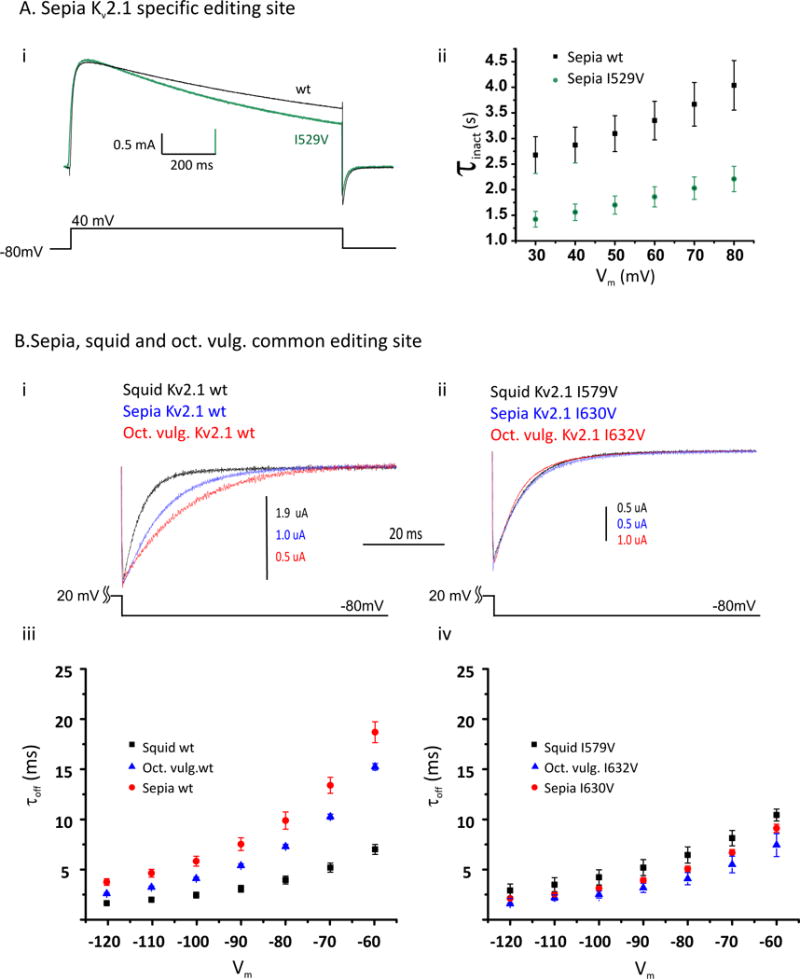
(A) (i) Current traces resulting from a voltage step from −80 mV to 40 mV for the wt Sepia Kv2.1 and the same construct containing the sepia-specific I529V edit, lying within the 4th transmembrane domain (green), showing that I529V accelerates the rate of slow inactivation. (ii) Time constants for slow inactivation determined by fitting single exponentials to traces similar to those in panel (i) at different activating voltages (Vm).
(B) (i) Tail currents measured at a voltage (Vm) of −80mV, following an activating pulse of +20 mV for 25 ms. Traces are shown for the wt Kv2.1 channels from squid, sepia and Octopus vulgaris. (ii) Tail currents for the same channels edited at the shared I-to-V site in the 6th transmembrane span, following the same voltage protocol. (iii) Time constants from single exponential fits to tail currents obtained at various negative voltages (Vm) (following an activating pulse to 20 mV for 25 ms) show that the unedited channels close at distinct rates, (iv) but the edited versions close at similar rates. N = 5 ± s.e.m. for all data plotted in this figure.
