Abstract
Background
Although depression and smoking commonly co-occur, the mechanisms underpinning this association are poorly understood. One hypothesis is that depression promotes tobacco dependence, persistence and relapse by increasing sensitivity to acute negative mood and abstinence induced tobacco-seeking behavior.
Methods
Twenty nine daily smokers of >10 cigarettes per day, nine with major depression and 20 without, completed two laboratory sessions one week apart, smoking as normal prior to session 1 (sated session), and 6 hours abstinent prior to session 2 (abstinent session). In both sessions, tobacco-seeking was measured at baseline by preference to view smoking versus food images. Negative mood was then induced by negative ruminative statements and sad music, before tobacco-seeking was measured again at test.
Results
In the sated session, negative mood induction produced a greater increase in tobacco choice from baseline to test in depressed (p<.001, ηp2=.782) compared to non-depressed smokers (p=.045, ηp2=.216, interaction: p=.046, ηp2=.150). Abstinence also produced a greater increase in baseline tobacco choice between the sated and abstinent sessions in depressed (p=.002, ηp2=.771) compared to non-depressed smokers (p=.22, ηp2=.089, interaction: p=.023, ηp2=.189). These mood and abstinence induced increases in tobacco choice were positively associated with depression symptoms across the sample as a whole (ps≤.04, ηp2≥.159), and correlated with each other (r=.67, p<.001).
Conclusions
Current major depression or depression symptoms may promote tobacco dependence, persistence and relapse by increasing sensitivity to both acute negative mood and abstinence induced tobacco-seeking behavior. Treatments should seek to break the association between adverse states and smoking to cope.
Keywords: Depression, Smoking, Vulnerability, Mood induction, Abstinence
1. Introduction
Although there is some bidirectionality, depression is known to prospectively promote drug dependence, persistence and relapse (Briere et al., 2014; Felton et al., 2015; Hitsman et al., 2013). One mechanistic explanation for this association is that depressed individuals are more sensitive to a cluster of correlated adverse interoceptive-emotional states which trigger drug use to cope, thus increasing the risk of dependence, persistence and relapse (Hussong et al., 2011; Mathew et al., 2016). This cluster of adverse triggers for drug use could include several distinct states such as rumination, anger, hostility, anxiety, stress, anhedonia, fatigue, or cognitive decline. However, perhaps the clearest findings pertain to acute negative mood and abstinence states. Specifically, smokers with current sub-clinical depression symptoms are more sensitive to the motivational effect of negative mood induction on ad libitum smoking behavior (Fucito and Juliano, 2009). Likewise, smokers with depression symptoms are more sensitive to the negative effects of smoking abstinence on affective state (Audrain-McGovern et al., 2014; Leventhal et al., 2013), reward responsiveness (Pergadia et al., 2014) and cognitive performance (Ashare et al., 2014). Finally, smokers with anhedonic traits are more sensitive to the effect of smoking abstinence on craving and willingness to pay for cigarettes (Cook et al., 2004; Leventhal et al., 2014; Leventhal et al., 2009). However, smokers with a history of major depression are not more sensitive to either negative mood or abstinence induced effects on ad libitum smoking behavior (Perkins et al., 2010). What remains to be tested within this literature, is whether smokers with current major depression are more sensitive to the motivational effect of negative mood induction and smoking abstinence on tobacco-seeking behavior, and whether these two sensitivities are correlated. If these expected findings are obtained in the current laboratory study, they would support the claim that depressed individuals are at risk of dependence, persistence and relapse because they are more sensitive to a cluster of correlated adverse interoceptive-emotional triggers for drug use behavior. The implication would be that treatment must simultaneously address the cluster of adverse triggers to improve efficacy in depressed smokers.
2. Method
2.1 Participants and Procedures
Data were drawn from a larger laboratory study examining the relationship between psychological risk factors and smoking lapse. The mood induced tobacco-seeking task was administered first in both sessions, immediately following questionnaires, and so should not have been influenced by the subsequent protocol.
Eligible participants were females and males aged 18–65 years who smoked >10 cigarettes/day for at least 6 months, had a breath carbon monoxide (CO) reading of >10 parts-per-million (ppm), and who scored at least 18 on the Intolerance for Smoking Abstinence Discomfort Questionnaire (IDQ-S) (Sirota et al., 2010). Exclusion criteria included selfreported chronic medical illness or severe mental illness (i.e. Psychotic or Bipolar Disorder), and current use of nicotine replacement or tobacco products other than cigarettes.
Participants attended two sessions 1–2 weeks apart, which were scheduled in either the morning or afternoon based on participant preference. Participants were instructed to smoke as normal prior to the first (sated) session and abstain from smoking for at least six hours prior to the second (abstinent) session. CO was recorded at the outset of each session. To confirm abstinence at session 2, self-report was verified by expired CO reading of either a) <10 ppm (Benowitz, 2002), or b) less than half of the baseline value, such that heavy smokers for whom a 6-hour abstinence period may not be sufficient to reach the 10 ppm cut-off value could be included. Participants were paid $60 for completing both sessions and provided informed consent at the start of session 1. All study procedures were approved by the appropriate Institutional Review Board.
2.2 Depression measures
Current major depression status was evaluated by the MINI International Neuropsychiatric interview for the DSM-5, version 7.0 (Sheehan et al., 1998). Trained research assistants administered the mood disorder algorithm (i.e., Major Depressive Episode, [Hypo]manic Episode, and Psychotic Disorders modules) to ascertain depression status. Those who endorsed a current (i.e., past 2 weeks) major depressive episode were classified as depressed smokers, while those who did not meet criteria for this disorder were classified as non-depressed. The Beck Depression Inventory-II (Beck et al., 1996) was administered to assess severity of depressive symptoms.
2.3 Mood induced tobacco choice task
The tobacco choice task shown in Figure 1A has been validated, in being increased by negative versus positive mood induction (Hogarth et al., 2015), by smoking abstinence versus satiety (Hogarth, 2012; Hogarth and Chase, 2011; Hogarth et al., 2013) and by tobacco dependence severity, craving, cigarettes per day, and smoking days per week (Hogarth, 2012; Hogarth and Chase, 2011, 2012). At baseline, participants freely chose to enlarge a smoking or food thumbnail image with a left or right key press, over 32 trials. In each trial, a smoking and food thumbnail was presented randomly in the left or right position, sampled from a set of 28 of each image type. Following baseline choice, pre-induction subjective mood was measured by participants reporting the extent to which they currently felt five positive (Enthusiastic, Happy, Excited, Inspired, Alert) and five negative emotions (Jittery, Upset, Distressed, Sad, Irritable), randomly ordered, on a five point scale ranging from ‘not at all’ to ‘extremely’. Sad music was then played through headphones (Barber’s Adagio for Strings), and participants were instructed to carefully consider sixteen negative statements (e.g. ‘I don’t think things are ever going to get better’) randomly ordered (Hogarth et al., 2015). Post-induction subjective mood was then measured in the same way as before. The tobacco choice test comprised 32 trials identical to baseline, except that the sad music continued to play and a negative statement (randomly selected from the set of 16) was presented prior to each choice.
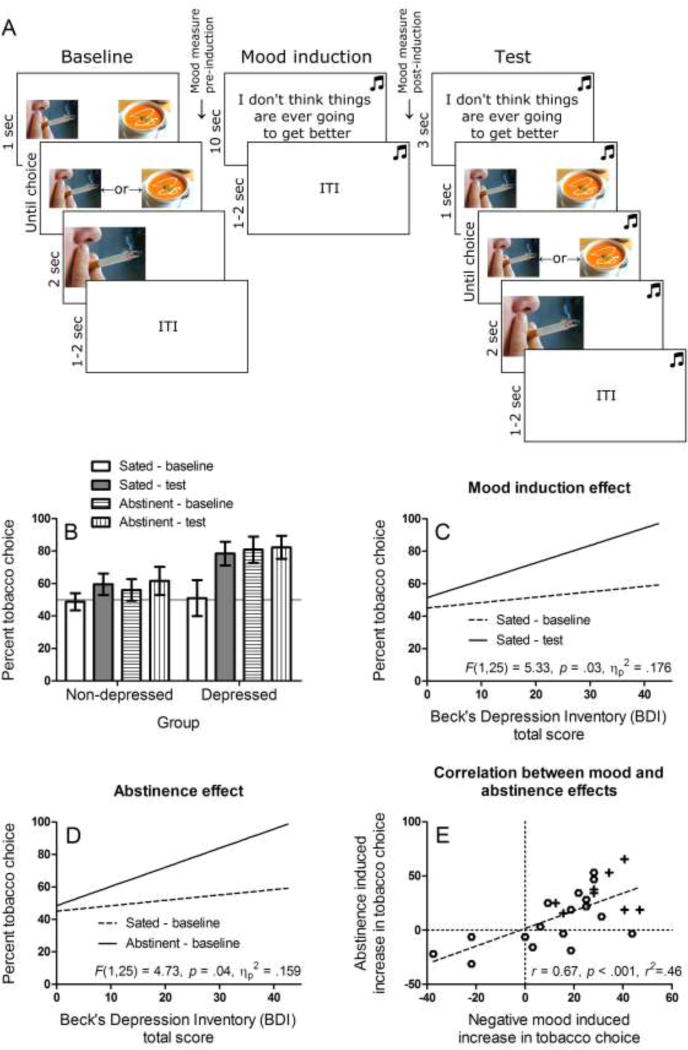
Figure 1.
A. Task used to test the effect of mood induction on tobacco choice, completed in both the sated and abstinent sessions.
B. Percent choice of tobacco versus food in the baseline and test blocks of the task, completed in the sated and abstinent sessions. Smokers with major depression showed a greater increase in tobacco choice between the sated-baseline and sated-test condition (mood induction effect), and between the sated-baseline and abstinent-baseline condition (abstinence effect), compared to non-depressed smokers.
C. Regression slopes relating depression symptoms (BDI-II) across the sample as a whole to percent tobacco choice in the baseline and test blocks completed in the sated session (mood induction effect). The difference between slopes increased with BDI-II scores indicating greater sensitivity to negative mood induced tobacco-seeking. The F statistic reports this interaction.
D. Regression slopes relating depression symptoms (BDI-II) across the sample as a whole to percent tobacco choice in the baseline blocks of the sated and abstinent session (abstinence effect). The difference between slopes increased with BDI-II scores indicating greater sensitivity to abstinence induced tobacco-seeking. The F statistic reports this interaction.
E. Correlation between the mood induction effect (sated-test minus sated-baseline) and the abstinence effect (abstinent-baseline minus sated-baseline) on tobacco choice across the sample as a whole. Symbols denote depressed (+) and non-depressed (O) smokers.
3. Results
3.1 Participants
Two participants were excluded for choosing tobacco in less than 1% of trials in both sessions, leaving 27 participants (the next lowest tobacco choice was 13%). Depressed and non-depressed groups differed with respect to BDI-II scores, but were matched with respect to other variables, including gender (Table 1).
Table 1.
Data collected from depressed and non-depressed smokers. BDI-II = Beck’s Depression Inventory-II. BDI-II scores 0–13 indicate minimal depression, scores 14–19 indicate mild depression, scores 20–28 indicate moderate depression, and scores 29–63 indicate severe depression.
| Group | |||
|---|---|---|---|
| Depressed (n=9) M (SD, range) |
Non-depressed (n=18) M (SD, range) |
p | |
| Age | 44.1 (11.4, 26–64) | 45.7 (11.4, 26–61) | .70 |
| Gender ratio (M/F) | 6/3 | 13/5 | .77 |
| Cigarettes per day | 18.1 (10.5, 8–40) | 14.3 (4.9, 7–25) | .45 |
| Age of smoking onset | 15.4 (2.3, 12–20) | 19.0 (5.5, 12–32) | .09 |
| BDI-II total score | 22.0 (3.0, 6–43) | 9.2 (8.6, 0–32) | .01* |
| Verification of abstinence | |||
| Breath CO sated session | 18.5 (7.3, 10.0–35.0) | 16.4 (5.3, 10.0–25.5) | See text |
| Breath CO abstinent session | 7.4 (4.3, 2.0–16.5) | 5.9 (3.1, 0–10.5) | |
| Minutes since smoking sated session | 32.9 (25.2, 12– 81) | 22.6 (20.9, 1–80) | |
| Minutes since smoking abstinent session | 614.4 (174.3, 367–990) | 769.3 (510.9, 375–2675) | |
| Verification of mood induction | |||
| + Mood pre-ind sated session | 2.4 (.7, 1.2–3.4) | 3.2 (1.1, 1.4–5.0) | See text |
| + Mood post-ind sated session | 1.8 (.7, 1.0–3.2) | 2.9 (1.2, 1.2–5.0) | |
| + Mood pre-ind abstinent session | 2.4 (.9, 1.0–3.6) | 3.3 (.9, 1.8–5.0) | |
| + Mood post-ind abstinent session | 2.1 (1.1, 1.0–3.6) | 3.1 (1.2, 1.2–5.0) | |
| − Mood pre-ind sated session | 2.7 (.6, 1.6–3.6) | 2.0 (.9, 1.0–3.6) | |
| − Mood post-ind sated session | 2.9 (.9, 1.4–4.0) | 2.0 (1.0, 1.0–4.8) | |
| − Mood pre-ind abstinent session | 2.7 (1.0, 1.6–4.4) | 2.1 (1.0, 1.0–4.8) | |
| − Mood post-ind abstinent session | 2.8 (1.0, 1.4–4.6) | 2.0 (1.0, 1.0–4.6) | |
CO = Carbon monoxide. Session 1 = smoking sated, session 2 = smoking abstinent. + Mood = positive mood measure. − Mood = negative mood measure. Pre-ind = pre-mood induction, post-ind = post-mood induction.
significant group contrast.
3.2 Verification of abstinence
ANOVA with CO scores (Table 1) revealed a main effect of session, F(1,25)=122.66, p<.001, ηp2=.831, but no effect of group or group by session interaction, Fs<1. At session 2, all participants reported abstinence for 6 hours or more, confirmed by CO values less than 10 ppm or half the session 1 value. ANOVA with minutes since smoking also revealed a main effect of session, F(1,25)=58.64, p<.001, ηp2=.701, and no effect of group or group by session interaction, Fs<1. Thus, the abstinence instructions in session 2 were successful.
3.3 Verification of mood induction
ANOVA with positive mood scores (averaged across the five positive words), shown in Table 1, revealed a main effect of block (pre-induction, post-induction), F(1,25)=4.98, p=.03, ηp2=.166, and group, F(1,25)=7.38, p=.01, ηp2=.228, but no other effects or interactions. ANOVA with mean negative mood scores (averaged across the five negative words) revealed a main effect of group, F(1,25)=5.69, p=.02, ηp2=.186, but no other effects or interactions. Thus, mood induction decreased positive mood, did not change negative mood, and depressed smokers scored lower on positive mood and higher on negative mood versus non-depressed smokers.
3.4 Tobacco choice
3.4.1 Depressed and non-depressed group
Figure 1B shows the percent choice of tobacco in the baseline and test blocks of the sated and abstinent sessions. ANOVA on these data yielded a significant interaction between group, session and block, F(1,25)=6.85, p=.01, ηp2=.215, Power=.71. Breakdown of this interaction indicated that there was a significant group by block interaction in the sated session, F(1,25)=4.41, p=.046 ηp2=.150, Power=.52, but not the abstinent session, F(1,25)=0.37, p=.55 ηp2=.015. Furthermore, there was a significant interaction between group and session in the baseline data, F(1,25)=5.83, p=.023 ηp2=.189, Power=.64, but not the test data, F(1,25)=0.03, p=.85 ηp2=.001. Within the depressed group, there was a significant effect of session, F(1,8)=8.64, p=.02, ηp2=.519, Power=.73, block, F(1,8)=17.03, p=.003, ηp2=.680, Power=.95, and session by block interaction, F(1,8)=18.38, p=.003, ηp2=.697, Power=.96. By contrast, within the non-depressed group there was no effect of session F(1,17)=0.81, p=.38, ηp2=.045, block, F(1,17)=4.11, p=.06, ηp2=.195, or session by block interaction, F(1,17)=1.21, p=.29, ηp2=.066. Specific contrasts within the depressed group revealed a significant effect of block (mood induction) in the sated session, F(1,8)=28.66, p=.001, ηp2=.782, Power=.99, but not in the abstinent session, F(1,8)=0.12, p=.74, ηp2=.014. Furthermore, there was an effect of session (abstinence) on tobacco choice at baseline, F(1,8)=19.67, p=.002, ηp2=.711, Power=.97, but not test, F(1,8)=0.38, p=.56, ηp2=.045. Specific contrasts within the non-depressed group revealed a significant effect of block in the sated session F(1,17)=4.69, p=.045, ηp2=.216, Power=.53, but not the abstinent session, F(1,17)=1.64, p=.22, ηp2=.088, and nor was there an effect of session in baseline, F(1,17)=1.65, p=.22, ηp2=.089, or test data, F(1,17)=0.13, p=.72, ηp2=.008.
3.4.2 Depression symptoms
ANCOVAs tested whether the mood and abstinence effects increased with depression symptom scores (BDI-II) across the sample as a whole. Figure 1C shows that the increase in tobacco choice from baseline to test in the sated session (mood induction effect) increased as a function of BDI scores, F(1,25)=5.33, p=.03, ηp2=.176, Power=.60. Furthermore, Figure 1D shows that the increase in tobacco choice between the baseline tobacco choice measured in the sated and abstinent sessions (abstinence effect) also increased as a function of BDI scores, F(1,25)=4.73, p=.04, ηp2=.159, Power=.55. Finally, Figure 1E shows that the mood and abstinence effects on tobacco choice were correlated r=0.67, p < .001, r2=.46. Note that exploratory analysis of the gender variable yielded no significant effects or interactions (result available upon request). Finally, when baseline breath CO and cigarettes smoked per day were entered as covariates into the analyses above, these variables did not show any significant effects or interactions, or change the status of the significant effects and interactions reported above.
4. Discussion
The study showed that smokers who met criteria for a diagnosis of current major depression or had current depression symptoms were more sensitive to both acute negative mood and abstinence induced priming of tobacco-seeking behaviour, and these two sensitivities were highly correlated. The study demonstrated sensitivities in clinical depression which had previously only been associated with sub-clinical depression and hedonic traits (Audrain-McGovern et al., 2014; Cook et al., 2004; Fucito and Juliano, 2009; Leventhal et al., 2013; Leventhal et al., 2014; Leventhal et al., 2009; Perkins et al., 2010). As individual differences in sensitivity to negative mood/stress induced craving (Back et al., 2010; Brady et al., 2006; Cooney et al., 1997; Higley et al., 2011; Sinha et al., 2011; Sinha et al., 2006) and abstinence induced craving (Aguirre et al., 2015; Ferguson et al., 2006; McCarthy et al., 2008; Piper et al., 2008; Zuo et al., 2016) predict a higher risk of relapse, these sensitivities arguably underpin dependence, persistence and relapse, and should be targeted to improve treatment outcomes.
Depressed smokers’ greater sensitivity to both mood and abstinence induced tobacco-seeking (and the strong correlation between these effects) can be explained in two ways. First, the negative mood induction and abstinence manipulation might have produced a common adverse state which motivated tobacco-seeking in both cases. Anhedonia has been suggested to underpin tobacco dependence transdiagnostically (Leventhal and Zvolensky, 2015) and could have motivated tobacco-seeking in both conditions. Indeed, the mood induction procedure did not change negative affect, but rather, significantly reduced positive affect, perhaps suggesting that it induced a state of anhedonia. Furthermore, tobacco abstinence produces greater anhedonia in smokers with a history of depression (Pergadia et al., 2014). Thus, anhedonia could have been the singular adverse state that motived tobacco-seeking in both the negative mood and abstinence conditions. The second explanation is that depressed smokers are more sensitive to a wide range of correlated adverse triggers for smoking. In fact, a wide range of adverse states (in addition to negative affect and abstinence) have been shown to augment tobacco motivation including stress/anxiety (Owens et al., 2014), rumination (Dvorak et al., 2011), anger/hostility (Quinn et al., 2014; Zuo et al., 2016), cognitive dysfunction (Hall et al., 2015), fatigue/sleepiness (Hamidovic and de Wit, 2009) and reward hyposensitivity (Peechatka et al., 2015). Although it remains to be tested whether depressed smokers are more sensitive to all of these adverse triggers, our finding that they are more sensitive to both mood induction and abstinence suggests this may be the case. If depressed smokers were more sensitive to a wide a range of adverse triggers, this could be due to generalisation between adverse states based on experiential overlap (Baker et al., 2004; Wood et al., 1989), or because the adverse states all acquire motivational properties as a consequence of being concurrently mitigated by smoking (Pomerleau and Pomerleau, 1984).
If depressed individuals’ tobacco dependence is driven by sensitivity to a cluster of adverse triggers, then treating only one trigger should achieve only limited therapeutic efficacy. Indeed, existing therapies which treat negative mood (Hartmann-Boyce et al., 2014) or withdrawal symptoms (Ferguson et al., 2006; McCarthy et al., 2008; Piper et al., 2008) achieve only modest efficacy. An important adjunct may come from a novel cognitive behavioral approach which first ascertains individuals’ range of adverse triggers for drug use, and then challenges their beliefs that drug use helps cope with these adverse states, thus treating multiple triggers simultaneously. This technique produces superior treatment outcomes compared to standard treatment in drinkers or smokers with either anxiety disorder or who report high coping motives (Anker et al., 2016; Bradizza et al., 2017; Kushner et al., 2013; Stasiewicz et al., 2013) suggesting that concurrent treatment of the trigger cluster may represent a progressive approach for depressed smokers.
To conclude, the current study provided evidence that current major depression and depression symptoms are associated with increased sensitivity to both negative mood and abstinence induced increases in tobacco-seeking behavior, and these two sensitivities were highly correlated. These findings fit with the view that depressed individuals’ risk of drug dependence, persistence and relapse is underpinned by a cluster of correlated adverse interoceptive-emotional triggers for smoking behavior. Improved therapeutic outcomes in depressed smokers may only be achieved by simultaneously treating the entire cluster of adverse triggers.
Highlights.
We examined factors underlying smoking maintenance in Major Depression (MD).
MD+ and MD− smokers completed assessments while smoking as usual and abstinent.
Sensitivity to negative reinforcement was assessed with a tobacco-seeking task.
Negative mood and abstinence prime greater tobacco-seeking for MD+ vs. MD− smokers.
Efficacious treatments should target both mechanisms among MD+ smokers.
Acknowledgments
Role of Funding Source. This research was supported by the National Institute on Drug Abuse of the National Institutes of Health under award F32 DA036947 (Amanda Mathew). Support was also provided by an Economic and Social Research Council (ERSC) PhD scholarship to Lorna Hardy. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Contributors. All three authors contributed materially to the design, running and reporting of the study. All authors have approved the final article.
Conflict of Interest. Brian Hitsman has served on a scientific advisory board for Pfizer and receives study medication and placebo free of charge from Pfizer for use in ongoing National Institutes of Health funded clinical trials.
References
- Aguirre C, Madrid J, Leventhal AM. Tobacco withdrawal symptoms mediate motivation to reinstate smoking during abstinence. J Abnorm Psychol. 2015;124:623–634. doi: 10.1037/abn0000060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anker JJ, Kushner MG, Thuras P, Menk J, Unruh AS. Drinking to cope with negative emotions moderates alcohol use disorder treatment response in patients with co-occurring anxiety disorder. Drug Alcohol Depend. 2016;159:93–100. doi: 10.1016/j.drugalcdep.2015.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashare R, Strasser AA, Wileyto EP, Cuevas J, Audrain-McGovern J. Cognitive deficits specific to depression-prone smokers during abstinence. Exp Clin Psychopharmacol. 2014;22:323–331. doi: 10.1037/a0037072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audrain-McGovern J, Wileyto EP, Ashare R, Cuevas J, Strasser AA. Reward and affective regulation in depression-prone smokers. Biol Psychiatry. 2014;76:689–697. doi: 10.1016/j.biopsych.2014.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Back SE, Hartwell K, DeSantis SM, Saladin M, McRae-Clark AL, Price KL, Moran-Santa Maria MM, Baker NL, Spratt E, Kreek MJ, Brady KT. Reactivity to laboratory stress provocation predicts relapse to cocaine. Drug Alcohol Depend. 2010;106:21–27. doi: 10.1016/j.drugalcdep.2009.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker TB, Piper ME, McCarthy DE, Majeskie MR, Fiore MC. Addiction motivation reformulated: An affective processing model of negative reinforcement. Psychol Rev. 2004;111:33–51. doi: 10.1037/0033-295X.111.1.33. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. BDI-II, Beck Depression Inventory: Manual. Harcourt Brace, San Antonio, Tex. Boston: Psychological Corp.; 1996. [Google Scholar]
- Benowitz NL. Biochemical verification of tobacco use and cessation. Nicotine Tob Res. 2002;4:149–159. doi: 10.1080/14622200210123581. [DOI] [PubMed] [Google Scholar]
- Bradizza CM, Stasiewicz PR, Zhuo Y, Ruszczyk M, Maisto SA, Lucke J, Brandon TH, Eiden RD, Slosman K, Giarratano P. Smoking cessation for pregnant smokers: Development and pilot test of an emotion regulation treatment (ERT) supplement for negative affect smokers. Nicotine and Tobacco Research. 2017 doi: 10.1093/ntr/ntw398. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady KT, Back SE, Waldrop AE, McRae AL, Anton RF, Upadhyaya HP, Saladin ME, Randall PK. Cold pressor task reactivity: Predictors of alcohol use among alcohol-dependent individuals with and without comorbid posttraumatic stress disorder. Alcoholism: Clinical and Experimental Research. 2006;30:938–946. doi: 10.1111/j.1530-0277.2006.00097.x. [DOI] [PubMed] [Google Scholar]
- Briere FN, Rohde P, Seeley JR, Klein D, Lewinsohn PM. Comorbidity between major depression and alcohol use disorder from adolescence to adulthood. Compr Psychiatry. 2014;55:526–533. doi: 10.1016/j.comppsych.2013.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook JW, Spring B, McChargue D, Hedeker D. Hedonic capacity, cigarette craving, and diminished positive mood. Nicotine Tob Res. 2004;6:39–47. doi: 10.1080/14622200310001656849. [DOI] [PubMed] [Google Scholar]
- Cooney NL, Litt MD, Morse PA, Bauer LO, Gaupp L. Alcohol cue reactivity, negative-mood reactivity, and relapse in treated alcoholic men. J Abnorm Psychol. 1997;106:243–250. doi: 10.1037//0021-843x.106.2.243. [DOI] [PubMed] [Google Scholar]
- Dvorak R, Simons J, Wray T. Impulsivity moderates the association between depressive rumination and number of quit attempt failures by smokers. Addiction Research & Theory. 2011;19:283–288. [Google Scholar]
- Felton JW, Kofler MJ, Lopez CM, Saunders BE, Kilpatrick DG. The emergence of co-occurring adolescent polysubstance use and depressive symptoms: A latent growth modeling approach. Development and Psychopathology. 2015;27:1367–1383. doi: 10.1017/S0954579414001473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson SG, Shiffman S, Gwaltney CJ. Does reducing withdrawal severity mediate nicotine patch efficacy? A randomized clinical trial. J Consult Clin Psychol. 2006;74:1153–1161. doi: 10.1037/0022-006X.74.6.1153. [DOI] [PubMed] [Google Scholar]
- Fucito LM, Juliano LM. Depression moderates smoking behavior in response to a sad mood induction. Psychology of Addictive Behaviors. 2009;23:546–551. doi: 10.1037/a0016529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall FS, Der-Avakian A, Gould TJ, Markou A, Shoaib M, Young JW. Negative affective states and cognitive impairments in nicotine dependence. Neurosci Biobehav Rev. 2015;58:168–185. doi: 10.1016/j.neubiorev.2015.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamidovic A, de Wit H. Sleep deprivation increases cigarette smoking. Pharmacol Biochem Behav. 2009;93:263–269. doi: 10.1016/j.pbb.2008.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann-Boyce J, Stead LF, Cahill K, Lancaster T. Efficacy of interventions to combat tobacco addiction: Cochrane update of 2013 reviews. Addiction. 2014;109:1414–1425. doi: 10.1111/add.12633. [DOI] [PubMed] [Google Scholar]
- Higley A, Crane N, Spadoni A, Quello S, Goodell V, Mason B. Craving in response to stress induction in a human laboratory paradigm predicts treatment outcome in alcohol-dependent individuals. Psychopharmacology. 2011;218:121–129. doi: 10.1007/s00213-011-2355-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitsman B, Papandonatos GD, McChargue DE, DeMott A, Herrera MJ, Spring B, Borrelli B, Niaura R. Past major depression and smoking cessation outcome: a systematic review and meta-analysis update. Addiction. 2013;108:294–306. doi: 10.1111/add.12009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogarth L. Goal-directed and transfer-cue-elicited drug-seeking are dissociated by pharmacotherapy: Evidence for independent additive controllers. J Exp Psychol: Anim Behav Processes. 2012;38:266–278. doi: 10.1037/a0028914. [DOI] [PubMed] [Google Scholar]
- Hogarth L, Chase HW. Parallel goal-directed and habitual control of human drug-seeking: Implications for dependence vulnerability. J Exp Psychol: Anim Behav Processes. 2011;37:261–276. doi: 10.1037/a0022913. [DOI] [PubMed] [Google Scholar]
- Hogarth L, Chase HW. Evaluating psychological markers for human nicotine dependence: Tobacco choice, extinction, and Pavlovian-to-instrumental transfer. Exp Clin Psychopharmacol. 2012;20:213–224. doi: 10.1037/a0027203. [DOI] [PubMed] [Google Scholar]
- Hogarth L, Field M, Rose AK. Phasic transition from goal-directed to habitual control over drug-seeking produced by conflicting reinforcer expectancy. Addict Biol. 2013;18:88–97. doi: 10.1111/adb.12009. [DOI] [PubMed] [Google Scholar]
- Hogarth L, He Z, Chase HW, Wills AJ, Troisi J, II, Leventhal AM, Mathew AR, Hitsman B. Negative mood reverses devaluation of goal-directed drug-seeking favouring an incentive learning account of drug dependence. Psychopharmacology. 2015;232:3235–3247. doi: 10.1007/s00213-015-3977-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussong AM, Jones DJ, Stein GL, Baucom DH, Boeding S. An internalizing pathway to alcohol use and disorder. Psychology of Addictive Behaviors. 2011;25:390–404. doi: 10.1037/a0024519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushner MG, Maurer EW, Thuras P, Donahue C, Frye B, Menary KR, Hobbs J, Haeny AM, Van Demark J. Hybrid cognitive behavioral therapy versus relaxation training for co-occurring anxiety and alcohol disorder: A randomized clinical trial. J Consult Clin Psychol. 2013;81:429–442. doi: 10.1037/a0031301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal AM, Ameringer KJ, Osborn E, Zvolensky MJ, Langdon KJ. Anxiety and depressive symptoms and affective patterns of tobacco withdrawal. Drug Alcohol Depend. 2013;133:324–329. doi: 10.1016/j.drugalcdep.2013.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal AM, Trujillo M, Ameringer KJ, Tidey JW, Sussman S, Kahler CW. Anhedonia and the relative reward value of drug and nondrug reinforcers in cigarette smokers. J Abnorm Psychol. 2014;123:375–386. doi: 10.1037/a0036384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal AM, Waters AJ, Kahler CW, Ray LA, Sussman S. Relations between anhedonia and smoking motivation. Nicotine Tob Res. 2009;11:1047–1054. doi: 10.1093/ntr/ntp098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal AM, Zvolensky MJ. Anxiety, depression, and cigarette smoking: A transdiagnostic vulnerability framework to understanding emotion–smoking comorbidity. Psychol Bull. 2015;141:176–212. doi: 10.1037/bul0000003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew AR, Hogarth L, Leventhal AM, Cook JW, Hitsman B. Cigarette smoking and depression comorbidity: systematic review and proposed theoretical model. Addiction. 2016 doi: 10.1111/add.13604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy DE, Piasecki TM, Lawrence DL, Jorenby DE, Shiffman S, Baker TB. Psychological mediators of bupropion sustained-release treatment for smoking cessation. Addiction. 2008;103:1521–1533. doi: 10.1111/j.1360-0443.2008.02275.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens MM, Ray LA, MacKillop J. Behavioral economic analysis of stress effects on acute motivation for alcohol. J Exp Anal Behav. 2014;103:77–86. doi: 10.1002/jeab.114. [DOI] [PubMed] [Google Scholar]
- Peechatka AL, Whitton AE, Farmer SL, Pizzagalli DA, Janes AC. Cigarette craving is associated with blunted reward processing in nicotine-dependent smokers. Drug Alcohol Depend. 2015;155:202–207. doi: 10.1016/j.drugalcdep.2015.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pergadia ML, Der-Avakian A, D'Souza MS, Madden PAF, Heath AC, Shiffman S, Markou A, Pizzagalli DA. Association between nicotine withdrawal and reward responsiveness in humans and rats. JAMA psychiatry. 2014;71:1238–1245. doi: 10.1001/jamapsychiatry.2014.1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins KA, Karelitz JL, Giedgowd GE, Conklin CA, Sayette MA. Differences in negative mood-induced smoking reinforcement due to distress tolerance, anxiety sensitivity, and depression history. Psychopharmacology. 2010;210:25–34. doi: 10.1007/s00213-010-1811-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper ME, Federmen EB, McCarthy DE, Bolt DM, Smith SS, Fiore MC, Baker TB. Using mediational models to explore the nature of tobacco motivation and tobacco treatment effects. J Abnorm Psychol. 2008;117:94–105. doi: 10.1037/0021-843X.117.1.94. [DOI] [PubMed] [Google Scholar]
- Pomerleau OF, Pomerleau CS. Neuroregulators and the reinforcement of smoking: Towards a biobehavioral explanation. Neurosci Biobehav Rev. 1984;8:503–513. doi: 10.1016/0149-7634(84)90007-1. [DOI] [PubMed] [Google Scholar]
- Quinn A, Sekimura S, Pang R, Trujillo M, Kahler CW, Leventhal AM. Hostility as a predictor of affective changes during acute tobacco withdrawal. Nicotine Tob Res. 2014;16:335–342. doi: 10.1093/ntr/ntt151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC. The Mini-International Neuropsychiatric Interview (M.I.N.I.): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(Suppl 20):22–33. quiz 34–57. [PubMed] [Google Scholar]
- Sinha R, Fox HC, Hong K, Hansen J, Tuit K, Kreek M. Effects of adrenal sensitivity, stress- and cue-induced craving, and anxiety on subsequent alcohol relapse and treatment outcomes. Archives of General Psychiatry. 2011;68:942–952. doi: 10.1001/archgenpsychiatry.2011.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Garcia M, Paliwal P, Kreek M, Rounsaville BJ. Stress-induced cocaine craving and hypothalamic-pituitary-adrenal responses are predictive of cocaine relapse outcomes. Archives of General Psychiatry. 2006;63:324–331. doi: 10.1001/archpsyc.63.3.324. [DOI] [PubMed] [Google Scholar]
- Sirota AD, Rohsenow DJ, Mackinnon SV, Martin RA, Eaton CA, Kaplan GB, Monti PM, Tidey JW, Swift RM. Intolerance for Smoking Abstinence Questionnaire: psychometric properties and relationship to tobacco dependence and abstinence. Addict Behav. 2010;35:686–693. doi: 10.1016/j.addbeh.2010.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stasiewicz PR, Bradizza CM, Schlauch RC, Coffey SF, Gulliver SB, Gudleski GD, Bole CW. Affect regulation training (ART) for alcohol use disorders: Development of a novel intervention for negative affect drinkers. J Subst Abuse Treat. 2013;45:433–443. doi: 10.1016/j.jsat.2013.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood DM, Laraby PR, Lal H. A pentylenetetrazol-like stimulus during cocaine withdrawal: Blockade by diazepam but not haloperidol. Drug Dev Res. 1989;16:269–276. [Google Scholar]
- Zuo Y, Rabinovich NE, Gilbert DG. Negative affect subtypes and craving differentially predict long-term cessation success among smokers achieving initial abstinence. Psychopharmacology. 2016:1–11. doi: 10.1007/s00213-016-4509-1. [DOI] [PMC free article] [PubMed] [Google Scholar]