Figure 5. Treatment of xenogeneic GVHD with MEMs allow for increased survival & inhibit T cells proliferation in vitro.
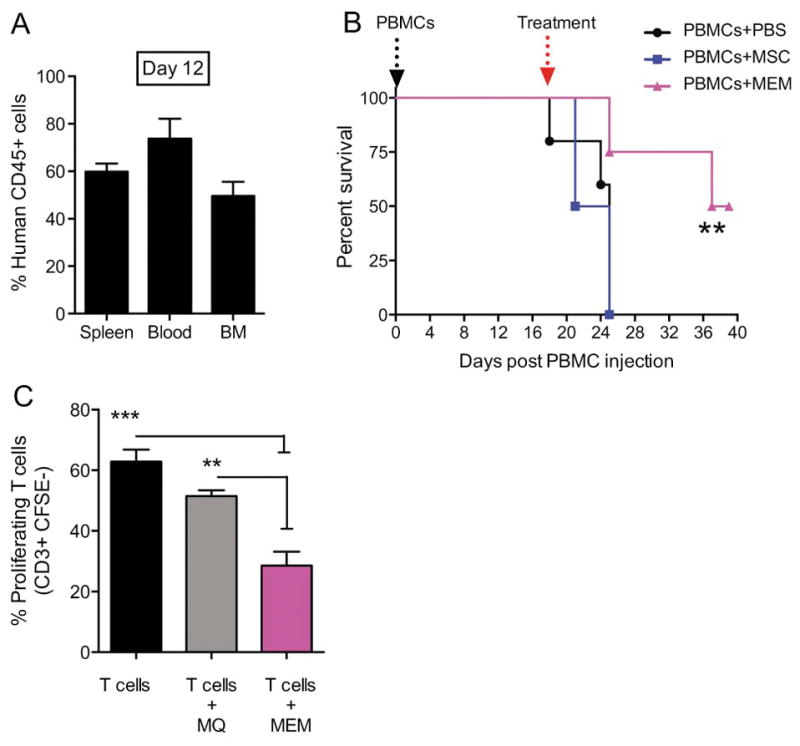
(A) Day +0, NSG mice received 30x106 PBMCs i.v to induce a xenogeneic GVHD in the absence of total body irradiation. (A) On Day +12 post transplant, human CD45+ engraftment in spleen, blood and bone marrow (BM) was assessed. (B) On day +18, when mice showed clinical evidence of GVHD, mice were randomized to receive PBS, 5x105 MSCs or MEMs i.v to treat GVHD and monitored for survival. N= 5 mice/group, one representative experiment of 3 performed. Survival curves compared by log rank analysis, **P<0.001 (C) Allogeneic CD3+ sorted CFSE-labeled T cells were cultured in triplicate with anti-CD3 and anti-CD28 antibodies either alone (T cells), with MØ (T cells + MØ), or MEM (T cells + MEM) at a 2:1 ratio for 5 days. Cells were then collected from each group and stained for CD3+ to determine the percentage of proliferating cells, which are CD3+CFSE−. Mean ± SEM calculated by one-way ANOVA.
