Figure 3.
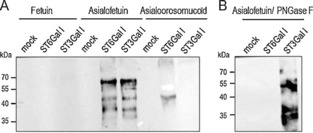
Enzymatic activity of Δ56ST6Gal I and Δ56ST3Gal I with CMP‐SiaNAl as a donor. A) Enzymatic reactions were performed for 4 h with 8.5 μL of the culture medium of transfected HEK293 cells containing either no enzyme (mock) or recombinant Δ56ST6Gal I (ST6Gal I) or Δ56ST3Gal I (ST3Gal I) and 50 μg of native fetuin, or desialylated fetuin (asialofetuin), or asialoorosomucoid (acceptor), and 100 μm freshly prepared CMP‐SiaNAl (conditions as described in Experimental Section). Covalent ligation of the SiaNAlkyne moiety with azido‐biotin was carried out by click chemistry. Re‐sialylated proteins were separated by SDS‐PAGE (8 %) and visualized by western blotting with peroxidase‐conjugated anti‐biotin antibody (16 ng mL−1). B) Specificity of Δ56ST3Gal I or Δ56ST6Gal I was assessed by PNGase F treatment of 20 μg of resialylated fetuin prior to click chemistry and western blot analysis. Then, covalent ligation of the SiaNAlkyne moiety with azido‐biotin and SDS PAGE were carried out. The signal previously detected on resialylated fetuin disappears upon PNGase F treatment of samples sialylated with Δ56ST6Gal I, whereas the signal remains for fetuin samples resialylated with Δ56ST3Gal I.
