Figure 5.
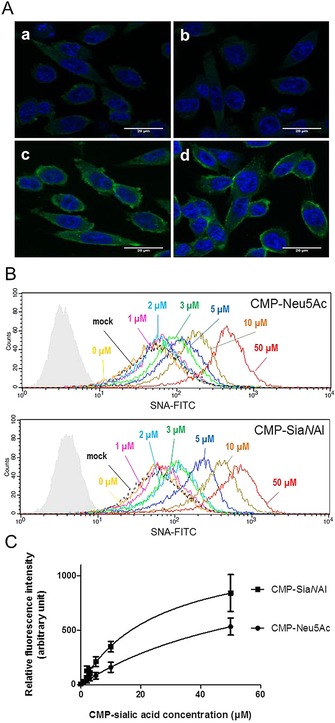
Δ56ST6Gal I activity in vivo using CHO Lec2 cells. A) Confocal microscopy images show labeling of cells using Δ56ST6Gal I. HEK293 cell culture medium (200 μL) expressing recombinant Δ56ST6Gal I enzyme (b, c, d) or no enzyme (a, mock) incubated for 2 h with CHO Lec2 cells and 20 μm CMP‐Neu5Ac (c) or CMP‐SiaNAl (d). CHO Lec2 cells were fixed in 4 % paraformaldehyde and treated with FITC‐SNA lectin, (1/75 e). Nuclei are stained with DAPI (blue). B) Flow cytometry analysis of CHO Lec2 cells after exogenous labeling with 300 μL of medium from HEK293 cells expressing Δ56ST6Gal I (or no enzyme, mock) and CMP‐Neu5Ac or CMP‐SiaNAl (0–50 μm, as indicated). Incubations were carried out for 2 h, and sialylated glycoconjugates were detected with FITC‐SNA lectin (1/100 e) and analyzed by flow cytometry. Autofluorescence control is shown in pale gray. No variation in fluorescence intensity was detected between CHO Lec2 cells incubated with no enzyme (dashed line, mock) or with enzyme but no CMP‐Sia donor (yellow, 0 μm). Significant variation in fluorescence was detected in CHO Lec2 cells incubated with cell culture medium expressing Δ56ST6Gal I and various amount of CMP‐Sias. Data are from one of three replicated experiments. C) Variation in fluorescence intensity (±SD) according to concentration of CMP‐Sias obtained in three independent experiments. These data indicate better detection of the transferred SiaNAl by the FITC‐SNA lectin.
