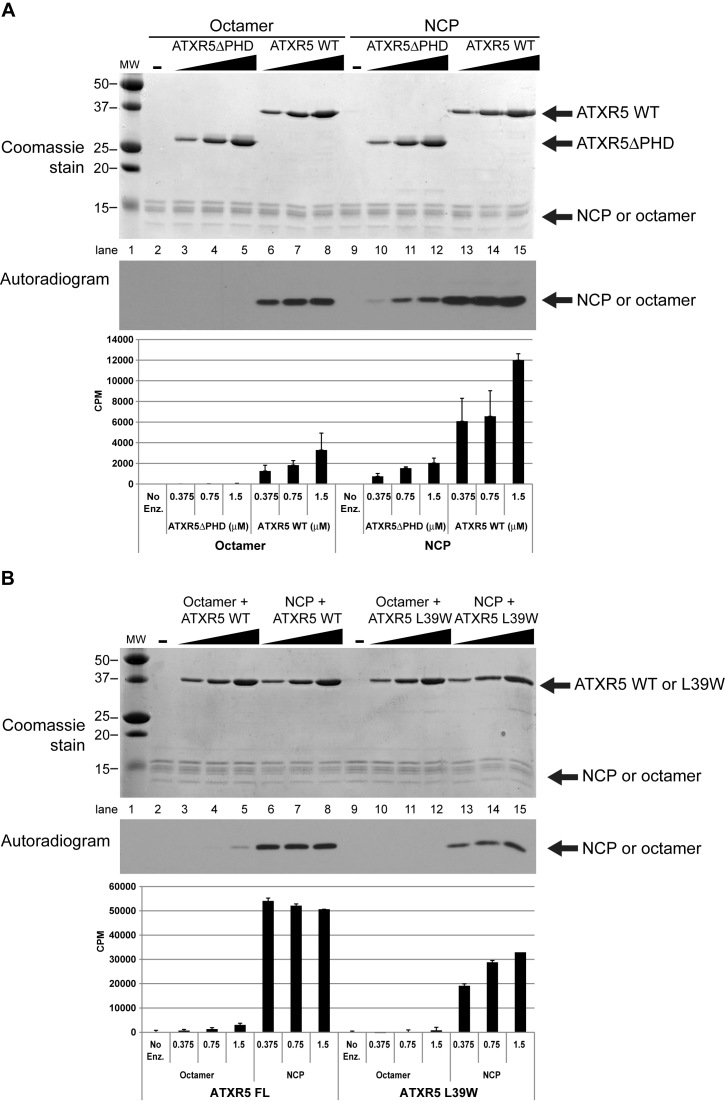
Figure 4.
ATXR5 activity is stronger on the NCP and with an intact PHD domain. KMTase assays were performed using histone octamers or NCP as substrate, using tritiated S-adenosyl-l-Methionine and varying amounts of WT or mutant enzyme. (A) Reactions performed with ATXR5 WT or a mutant in which the PHD domain has been deleted. Reaction products were separated by SDS-PAGE, gel extracted using isopropanol and radioactivity on histones was quantitated by scintillography. (B) Similar reactions were performed, this time comparing ATXR5 WT or L39W mutant. Reactions were stopped by spotting the reactions on P81 filter paper and quantified by scintillography.