Abstract
Objective:
Myoclonus, a common complication during intravenous induction with etomidate, is bothersome to both anesthesiologists and patients. This study explored the preventive effect of pretreatment with propofol on etomidate-related myoclonus.
Methods:
This was a prospective, double-blind, clinical, randomized controlled study. Totally, 363 patients who were scheduled for a short-duration, painless gastrointestinal endoscopy were divided into 5 groups. Four groups received 0 mg/kg (E group), 0.25 mg/kg (LPE group), 0.50 mg/kg (MPE group), or 0.75 mg/kg (HPE group) propofol pretreatment before etomidate anesthesia. Another group only received 1 to 2 mg/kg of propofol (P group) as anesthesia. The incidence and severity of myoclonus, patient circulation and respiratory status, and intraoperative and postoperative complications were recorded.
Results:
The incidence of myoclonus in the LPE group (26.8%), MPE group (16.4%), HPE group (14.9%), and P group (0) was lower than the E group (48.6%, P < .05). The incidence of grade 1, 2, and 3 of myoclonus in the LPE group, MPE group, HPE group, and P group was significantly lower than the E group, and that in the P group was lower than the LPE group (P < .05). The incidence of hypoxemia in the P group was higher than the E group, and the incidence of adverse events in the HPE group and P group was lower than the E group (P < .05).
Discussion:
Pretreatment with propofol was feasible for preventing etomidate-related myoclonus. Furthermore, as propofol dosage increased, its effect on reducing the incidence and severity of myoclonic movements induced by etomidate increased.
Keywords: etomidate, myoclonus, painless gastroscopy, propofol
1. Introduction
Etomidate is a short-acting, nonbarbiturate intravenous anesthetic agent used in clinical medicine since 1972.[1] It is suitable for anesthetizing patients with coronary artery disease and poor cardiac reserve owing to its characteristics, including rapid onset, stable induction, and minor effects on the circulatory and respiratory system. It has been widely used in anesthesia for critically ill patients and patients with cardiovascular disease.[2,3]
However, intravenous induction with etomidate is usually associated with myoclonus. Myoclonus can occur after induction with etomidate in 50% to 80% of patients without pretreatment,[4] and it increases muscular tension similar to spasms in patients with severe myoclonus. This phenomenon may lead to negative, unpredictable effects during an operation, as well as the subsequent outcome and prognosis, especially for patients with full stomachs or ocular trauma.[5] As shown in recent studies, pretreatment with neuromuscular blocking agent,[6] opioids,[7,8] dexmedetomidine,[9] midazolam,[10] low-dose ketamine,[11] gabapentin,[12] dezocine,[13] and magnesium sulfate[14] could prevent etomidate-related myoclonus. However, these drugs are associated with side effects such as excessive sedation, delayed recovery, and respiratory inhibition. Thus, it is essential to explore a new approach to inhibit etomidate-related myoclonus with few complications.
In a preliminary investigation, we found that pretreatment with propofol could inhibit the myoclonus induced by etomidate. Etomidate plus propofol had few effects on respiration and circulation in patients, and was safer and more effective than propofol alone.[15] However, the effect and the optimal dose of propofol to minimize side effects are not well established. Thus, this study investigated the effect of pretreatment with different doses of propofol on etomidate-related myoclonus in patients scheduled for painless gastroscopy.
2. Materials and methods
This study used a prospective, double-blind, clinical, randomized controlled design and was approved by the Harbin Medical University Institutional Ethics Committee. Each patient signed a written informed consent to receive anesthesia after being informed of the advantages and disadvantages of each anesthesia scheme. This manuscript adheres to the applicable Equator guidelines.
2.1. Patients
In total, 375 adult patients of both sexes, aged 18 to 80 years, American Society of Anesthesiologists status (ASA) of I or II, who were scheduled for an elective painless gastroscopy planned to last <30 minutes, were included in the study. Using a random number table, they were divided equally into 5 groups (n = 75 in each group) to receive propofol in different doses (0 mg/kg, 0.25 mg/kg, 0.50 mg/kg, or 0.75 mg/kg) combined with etomidate anesthesia. These groups were as follows: etomidate (E) group, low-dose propofol and etomidate (LPE) group, middle-dose propofol and etomidate (MPE) group, and high-dose propofol and etomidate (HPE) group, or propofol anesthesia only (P) group. Patients with abnormal cardiac, respiratory, hepatic or renal function, history of metabolic disease, neuromuscular system disease or psychotic disorder, anemia, or known hypersensitivity to emulsion were excluded. Female patients were not pregnant or parturient. None of the patients received any sedatives or analgesics 2 weeks before the operation.
2.2. Anesthesia and operation
All of the patients started to fast 8 hours before the operation and did not receive any preoperative medication. Upon arrival at the operating room, peripheral intravenous access was established, and oxygen (3 L/min) was administered via a nasal cannula. Noninvasive mean arterial pressure (MAP), heart rate (HR), electrocardiogram (ECG), and arterial oxygen saturation (SpO2) were monitored during anesthesia. An intravenous injection of 0.8 μg/kg fentanyl was first performed, and then an intravenous injection of 0.3 mg/kg lidocaine was administered 1 minute later. After an additional 2 minutes, patients in the E group, LPE group, MPE group, and HPE group received a slow intravenous infusion of 0.05 mL/kg saline, followed by 0 mg/kg, 0.25 mg/kg, 0.50 mg/kg, or 0.75 mg/kg propofol (Lot number: GK053, AstraZeneca, Italy) according to the anesthesia induction scheme. Ninety seconds later, 0.2 mg/kg etomidate (Lot number: 20110610, Jiangsu Nhwa Pharmaceutical Co Ltd., China) in the LPE, MPE, HPE groups and 0.3 mg/kg etomidate in the E group were administered within 60 seconds. Patients in the P group received a slow intravenous infusion of 0.05 mL/kg saline, followed by a 60-second infusion of 1 to 2 mg/kg propofol 90 seconds later. To maintain the anesthesia during the operation, 0.06 mg/kg etomidate in the E group, LPE group, MPE group, and HPE group and 0.50 mg/kg propofol in the P group was additionally administered every 4 minutes or when patients had a modified Bromage score of 5, as performed by Breen et al.[16] The gastroscopy was performed under standard clinical protocols. A 6-mg dose of ephedrine was injected intravenously when intraoperative systemic blood pressure decreased to <80 mmHg or <30% of the basic value. A 0.3-mg dose of atropine was administered intravenously when HR decreased to <50 bmp. Hypoxemia was determined when SpO2 <93%, and if the duration of hypoxemia was >30 seconds. Patients’ jaws were adjusted to improved ventilation, but if the hypoxemia did not improve, the patient was ventilated by simple respirator.
2.3. Measurement
Myoclonus was the primary focus of this study. Myoclonus occurred within 5 minutes after the etomidate injection was recorded by an anesthesiologist who was blind to the patients’ treatment. The severity of myoclonus was assessed according to a method by Doenicke et al,[17] and the intensity of myoclonic movement was graded as follows: 0 = no myoclonus, 1 = mild myoclonus, 2 = moderate myoclonus, and 3 = severe myoclonus. In addition, intraoperative vital signs and occurrences of adverse effects including injection pain, bucking, hiccups, headache, dizziness, nausea, vomiting, intraoperative awareness, and psychiatric symptoms were also monitored.
2.4. Statistics
Quantitative data were presented as the mean ± standard deviation (SD) and were compared using a 1-way analysis of variance. Qualitative data were compared using a χ2 test, rank-sum test, and Ridit analysis, and ordinal data were tested by Kruskall wallis first, and then analyzed using a Nemenyi test. A P < .05 was considered statistically significant. With the respect to the incidence of myoclonus, the sample size was calculated assuming α = 0.05 and β = 0.2 (power: 80%).
3. Results
Twelve patients were excluded because of operation duration >30 minutes, change of operation plan, delayed recovery from anesthesia after operation caused by low blood glucose (<2.8 mmol/L), assisted respiration after induction drug administration, and patients with a difficult airway that could affect their vital signs. The numbers of patients in the E group, LPE group, MPE group, HPE group, and P group were 72, 71, 73, 74, and 73, respectively. There was no significant difference among the 5 groups in age, weight, sex ratio, and ASA classification (Table 1).
Table 1.
Characteristics of patient.
3.1. Myoclonus
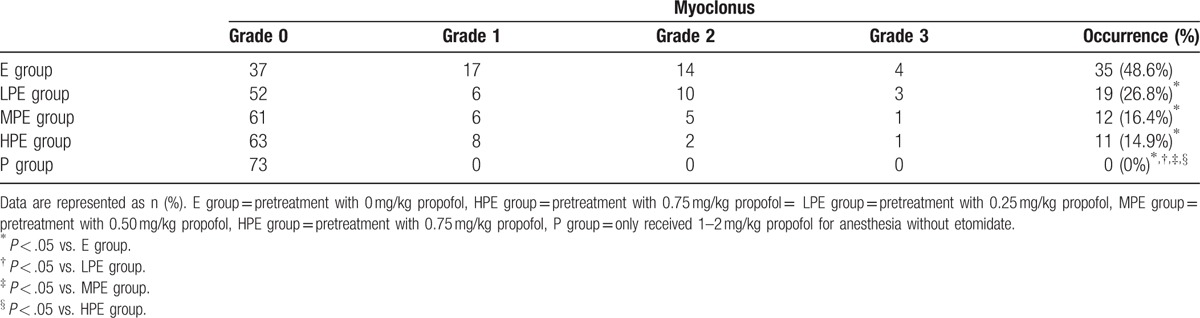
The patients in each group showed different patterns in the occurrence and severity of myoclonus (Table 2). The total incidence of myoclonus decreased as the dosage of propofol increased. The incidence of myoclonus in the LPE group (26.8%), MPE group (16.4%), HPE group (14.9%), and the P group (0) was lower than that in the E group (48.6%) (P < .05). Additionally, the incidence of myoclonus in the P group was lower than that in the LPE group, MPE group, and HPE group (P < .05). Furthermore, the incidence of grade 1, 2, and 3 myoclonus in the LPE group, MPE group, HPE group, and P group was significantly lower than that in the E group (P < .05), and the incidence of these grades in the P group was lower than that in the LPE group (P < .05)
Table 2.
Incidence and severity of myoclonus.
3.2. Circulation and respiration
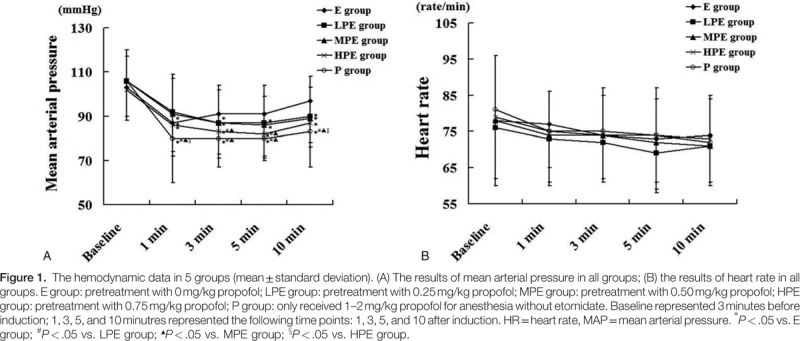
The MAP was measured at preinduction and 3, 5, and 10 minutes after induction. The MAP of patients in the P group strongly dropped in the first 3 minutes after induction, and became lower than the basal value, and then it restored gradually. The MAP in the P group and HPE group was significantly lower than that in the E group, LPE group, and MPE group (P < .05) (Fig. 1). Heart rate dropped in general. The incidence of hypoxemia after anesthesia induction was zero in the E group, 2.82% (2 patients) in the LPE group, 1.37% (1 patient) in the MPE group, 2.7% (2 patients) in the HPE group, and 6.85% (5 patients) in the P group. Additionally, the incidence of hypoxemia in the P group was significantly higher than that in the E group (P < .05).
3.3. Adverse events
In the E group, 2 patients experienced dizziness, vomiting, and nausea; 1 patient experienced bucking and 1 patient had psychiatric symptoms. In the LPE group, 3 patients experienced dizziness, vomiting, and nausea. In the MPE group, 1 patient experienced vomiting and nausea. These adverse effects were mild and transient and disappeared by the next day. No adverse events were observed in the HPE group or the P group. Furthermore, the incidence of adverse events in the HPE group and the P group was lower than that in the E group (P < .05).
4. Discussion
These data indicated that low dosage of propofol (0.25–0.75 mg/kg) pretreatment decreased the incidence of etomidate-related myoclonus, and the effect of myoclonus inhibition was more pronounced when combined with a dosage of propofol at 0.50 mg/kg and 0.75 mg/kg. However, the incidence of adverse events, including the inhibition of respiration and circulation, increased as the propofol dosage increased. Etomidate was a kind of intravenous anesthetics, which was suitable for frail patients with unstable hemodynamics, but had some side effects, including myoclonus and cortical hormone suppression.[3] Propofol was the most commonly used intravenous anesthetics with some disadvantages on hemodynamics. How to take the advantages and prevent the shortcoming of the 2 anesthetics were the primary tasks in the process of anesthesia induction. Etomidate, a short-acting imidazole-derivative anesthetic, can rapidly enter the brain and other blood-rich organs after intravenous injection and can provide hypnotic effects in one arm-brain circulation time.[18] Etomidate is associated with some transient complications such as injection pain, adrenal suppression, and myoclonus. Injection pain may result from local irritation caused by etomidate,[19] and adrenal suppression may result from reversible inhibition of 11-β-hydroxylase, which converts 11-deoxycortisol into cortisol.[20] However, the explicit mechanism of myoclonus development remains unclear.[11,21,22] In our study, the incidence of myoclonus in the etomidate group was 48.6%, which was close to the rate of 50% to 80% reported by Doenicke et al.[17] The slight difference might be because of the administration of 0.3 mg/kg lidocaine in this study, which could inhibit the myoclonus partially induced by etomidate.[23]
Several agents have been reported to reduce myoclonus associated with etomidate in varying degrees. However, the exact neurological mechanism of myoclonus reduced by etomidate is unclear. Some studies explored that myoclonic activity might be associated with disinhibition of subcortical structures owing to inhibition at the spinal level or cerebral cortex, rather than being associated with epilepsy.[17,22,24] Another study indicated that the pathways related to skeletal muscle control became more sensitive to spontaneous nerve transmissions once GABA neurons were disrupted, causing myoclonic muscle movements.[10] Recently, some authors found that etomidate could modulate the desensitization of recombinant β3α1δ/β3α1 GABAA receptors of the central nerve systerm.[25,26] Thus, we can deduce that etomidate suppressed the central nervous reticular activation system by interacting with GABAA receptors. By interrupting GABA neurons, the pathways associated with skeletal muscle control became more sensitive. These events ultimately led to myoclonic muscle contractions. Bai et al[27] found that propofol modulated GABAA receptor deactivation and desensitization to some extent. In this study, propofol pretreatment at a lower dosage (0.25–0.75 mg/kg) exerted inhibitory effects on myoclonus induced by etomidate. We inferred that propofol could have decreased the excitability of the central nervous system through the GABAA receptor and increased dopamine levels in the nucleus accumbens of the brain.[28] However, the exact mechanism needs to be further clarified.
Etomidate itself has little effect on the circulatory system[29] and respiratory depression caused by etomidate was relatively transient. Values only became significantly lower than the control values during the 2nd minute after the induction,[30] which may have resulted in an increase of approximately 15% of PaCO2 but unchanged PaO2.[31] In this trial, as the dosage of propofol increased, the probability of respiratory and cardiovascular depression also increased, which was similar to a previous study.[32] Numerous studies have found that propofol can reduce blood pressure by suppressing sympathetic activity, inhibiting myocardial contractility, and dilating blood vessels. However, an increase in propofol concentration within the therapeutic range causes a decrease in vascular-stressed volume without a change in CO.[33] Excessive dosage of propofol or rapid delivery can cause a decrease in respiratory frequency and tidal volume,[34] which may lead to respiratory depression and hypoxia. In this study, propofol pretreatment had little influence on respiration, possibly because the dosage used did not reach the threshold. Propofol can inhibit throat reflection and lead to apnea with the induction dosage indicated in the P group. Moreover, as mentioned above, the high-dose propofol pretreatment showed an impact on circulation. Therefore, the dosage of propofol should be balanced considering its advantages and disadvantages.
The dosage of anesthetics in this study was decided by the anesthesia depth, which was determined by propofol and etomidate interaction. Therefore, the propofol dose in the P group was higher than that in other groups, and the optimal dosage for each patient was individual, depending on the patient's status. Personalized medicine could have reduced the risk of hypoxia and excessive sedation in the P group. Therefore, just as Zhou et al[15] reported, etomidate plus propofol had few effects on respiration and circulation in patients undergoing gastroscopy and were safer and more effective than propofol alone.
Many drugs could inhibit etomidate-related myoclonus when administered in advance. However, pretreatment can cause adverse events such as prolonged recovery time induced by benzodiazepine; circulatory and respiratory depression and chest wall rigidity induced by opioids; and airway obstruction, reflux, and aspiration induced by muscle relaxants. Therefore, pretreatments should be limited to short durations and for minor operations. The ideal drugs used for pretreatment should be short-acting agents without significant effects on respiratory and circulatory parameters and no association with prolonged postoperative recovery time.[10] Thus, etomidate itself and low-dose propofol might be the ideal drugs for pretreatment. Aissaoui et al[35] found that pretreatment with a low dose (0.03 mg/kg) of etomidate at 60 seconds before induction with etomidate could effectively inhibit the development of myoclonus. In this study, we demonstrated that propofol pretreatment inhibited myoclonus induced by etomidate, and the pretreatment had no severe related side effects.
This clinical trial was performed for outpatients, and some information cannot be fully obtained. Previous studies showed that benzodiazepine medicine,[10] opioids,[9] and dexmedetomidine[7] could prevent etomidate-related myoclonus. These factors may influence the outcomes of this study. Therefore, to avoid the influence induced by the above-mentioned factors, this study excluded the patients with possible influencing factors. Maybe many uncertain factors were not excluded, and this was a limitation. There were other limitations of this study. First, the exact anesthesia depth was not monitored during anesthesia induction, and it was not clear whether the inhibition of myoclonus owing to propofol was related to the anesthesia depth; second, the specific mechanisms involved in the inhibitory effect of propofol pretreatment on myoclonus induced by etomidate were not explored. Finally, in the LPE, MPE, and HPE groups, the dose of etomidate was 0.2 mg/kg, whereas 0.3 mg/kg in the E group. The conventional induction dose of etomidate was 0.3 mg/kg, whereas the conventional induction dose of propofol was 1.5 to 2.5 mg/kg. There was an additive effect of the anesthesia depth when the 2 agents applied together. Therefore, to avoid a deeper anesthesia and some unnecessary complications, such as low blood pressure and respiratory inhibition, we chose 0.2 mg/kg etomidate in the mixed medicine groups.
Additionally, the degree of myoclonus was most grade 1 and 2, and rare grade 3, which could induce muscle pain, elevate serum potassium, brain metabolic rate, and intraocular pressure, and even increase the rate of aspiration.[36] Therefore, it was necessary to take some appropriate precautions to decrease the incidence of myoclonus. In this study, a low dose of propofol was pretreated and the dose of etomidate was decreased to 0.2 mg/kg from 0.3 mg/kg, which eventually decreased the severity and incidence rate of myoclonus. Thus, the clinical effects on patients were significant.
In conclusion, 0.50 mg/kg propofol pretreatment before the anesthesia induction with etomidate had little influence on hemodynamics, and had few side effects on patients, including myoclonus. Propofol was the ideal drug to inhibit the myoclonus, and decreased the induction dose of etomidate in patients scheduled for painless gastroscopy.
Footnotes
Abbreviations: ASA = American Society of Anesthesiologists status, ECG = electrocardiogram, HR = heart rate, MAP = mean arterial pressure, SpO2 = arterial oxygen saturation.
The authors report no conflicts of interest.
JL and RL contributed equally to this work.
References
- [1].Morgan M, Lumley J, Whitwam JG. Etomidate, a new water-soluble non-barbiturate intravenous induction agent. Lancet 1975;1:955–6. [DOI] [PubMed] [Google Scholar]
- [2].Song JC, Lu ZJ, Jiao YF, et al. Etomidate anesthesia during ERCP caused more stable haemodynamic responses compared with propofol: a randomized clinical trial. Int J Med Sci 2015;12:559–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Shah SB, Chowdhury I, Bhargava AK, et al. Comparison of hemodynamic effects of intravenous etomidate versus propofol during induction and intubation using entropy guided hypnosis levels. J Anaesthesiol Clin Pharmacol 2015;31:180–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Yates AM, Wolfson AB, Shum L, et al. A descriptive study of myoclonus associated with etomidate procedural sedation in the ED. Am J Emerg Med 2013;31:852–4. [DOI] [PubMed] [Google Scholar]
- [5].Hueter L, Schwarzkopf K, Simon M, et al. Pretreatment with sufentanil reduces myoclonus after etomidate. Acta Anaesthesiol Scand 2003;47:482–4. [DOI] [PubMed] [Google Scholar]
- [6].Choi JM, Choi IC, Jeong YB, et al. Pretreatment of rocuronium reduces the frequency and severity of etomidate-induced myoclonus. J Clin Anesth 2008;20:601–4. [DOI] [PubMed] [Google Scholar]
- [7].Wang J, Li QB, Wu YY, et al. Efficacy and safety of opioids for the prevention of etomidate-induced myoclonus: a meta-analysis. Am J Ther 2016;DOI: 10.1097/MJT.0000000000000404. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- [8].Ko BJ, Oh JN, Lee JH, et al. Comparison of effects of fentanyl and remifentanil on hemodynamic response to endotracheal intubation and myoclonus in elderly patients with etomidate induction. Korean J Anesthesiol 2013;64:12–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Luan HF, Zhao ZB, Feng JY, et al. Prevention of etomidate-induced myoclonus during anesthetic induction by pretreatment with dexmedetomidine. Braz J Med Biol Res 2015;48:186–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Hüter L, Schreiber T, Gugel M, et al. Low-dose intravenous midazolam reduces etomidate-induced myoclonus: a prospective, randomized study in patients undergoing elective cardioversion. Anesth Analg 2007;105:1298–302. [DOI] [PubMed] [Google Scholar]
- [11].Wu GN, Xu HJ, Liu FF, et al. Low-dose ketamine pretreatment reduces the incidence and severity of myoclonus induced by etomidate: a randomized, double-blinded, controlled clinical trial. Medicine (Baltimore) 2016;95:e2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Yılmaz Çakirgöz M, Demirel İ, Duran E, et al. Effect of gabapentin pretreatment on myoclonus after etomidate: a randomized, double-blind, placebo-controlled study. Braz J Anesthesiol 2016;66:356–62. [DOI] [PubMed] [Google Scholar]
- [13].He L, Ding Y, Chen H, et al. Dezocine pretreatment prevents myoclonus induced by etomidate: a randomized, double-blinded controlled trial. J Anesth 2015;29:143–5. [DOI] [PubMed] [Google Scholar]
- [14].Un B, Ceyhan D, Yelken B. Prevention of etomidate-related myoclonus in anesthetic induction by pretreatment with magnesium. J Res Med Sci 2011;16:1490–4. [PMC free article] [PubMed] [Google Scholar]
- [15].Zhou X, Li BX, Chen LM, et al. Etomidate plus propofol versus propofol alone for sedation during gastroscopy: a randomized prospective clinical trial. Surg Endosc 2016;30:5108–16. [DOI] [PubMed] [Google Scholar]
- [16].Breen TW, Shapiro T, Glass B, et al. Epidural anesthesia for labor in an ambulatory patient. Anesth Analg 1993;77:919–24. [DOI] [PubMed] [Google Scholar]
- [17].Doenicke AW, Roizen MF, Kugler J, et al. Reducing myoclonus after etomidate. Anesthesiology 1999;90:113–9. [DOI] [PubMed] [Google Scholar]
- [18].Nimmo WS, Miller M. Pharmacology of etomidate. Contemp Anesth Pract 1983;7:83–95. [PubMed] [Google Scholar]
- [19].Korttila K, Aromaa U. Venous complications after intravenous injection of diazepam, flunitrazepam, thiopentone and etomidate. Acta Anaesthesiol Scand 1980;24:227–30. [DOI] [PubMed] [Google Scholar]
- [20].Preziosi P, Vacca M. Adrenocortical suppression and other endocrine effects of etomidate. Life Sci 1988;42:477–89. [DOI] [PubMed] [Google Scholar]
- [21].Isitemiz I, Uzman S, Toptaş M, et al. Prevention of etomidate-induced myoclonus: which is superior: Fentanyl, midazolam, or a combination? A Retrospective comparative study. Med Sci Monit 2014;20:262–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Reddy RV, Moorthy SS, Dierdorf SF, et al. Excitatory effects and electroencephalographic correlation of etomidate, thiopental, methohexital, and propofol. Anesth Analg 1993;77:1008–11. [DOI] [PubMed] [Google Scholar]
- [23].Gultop F, Akkaya T, Bedirli N, et al. Lidocaine pretreatment reduces the frequency and severity of myoclonus induced by etomidate. J Anesth 2010;24:300–2. [DOI] [PubMed] [Google Scholar]
- [24].Modica PA, Tempelhoff R, White PF. Pro- and anticonvulsant effects of anesthetics (Part II). Anesth Analg 1990;70:433–44. [DOI] [PubMed] [Google Scholar]
- [25].Maldifassi MC, Baur R, Sigel E. Functional sites involved in modulation of the GABAA receptor channel by the intravenous anesthetics propofol, etomidate and pentobarbital. Neuropharmacology 2016;105:207–14. [DOI] [PubMed] [Google Scholar]
- [26].Liu K, Jounaidi Y, Forman SA, et al. Etomidate uniquely modulates the desensitization of recombinant (1(3( GABA(A) receptors. Neuroscience 2015;300:307–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Bai D, Pennefather PS, MacDonald JF, et al. The general anesthetic propofol slows deactivation and desensitization of GABA(A) receptors. J Neurosci 1999;19:10635–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Pain L, Gobaille S, Schleef C, et al. In vivo dopamine measurements in the nucleus accumbens after nonanesthetic and anesthetic doses of propofol in rats. Anesth Analg 2002;95:915–9. [DOI] [PubMed] [Google Scholar]
- [29].Harris CE, Murray AM, Anderson JM, et al. Effects of thiopentone, etomidate and propofol on the haemodynamic response to tracheal intubation. Anaesthesia 1988;43(suppl):32–6. [DOI] [PubMed] [Google Scholar]
- [30].Morgan M, Lumley J, Whitwam JG. Respiratory effects of etomidate. Br J Anaesth 1977;49:233–6. [DOI] [PubMed] [Google Scholar]
- [31].Colvin MP, Savege TM, Newland PE, et al. Cardiorespiratory changes following induction of anaesthesia with etomidate in patients with cardiac disease. Br J Anaesth 1979;51:551–6. [DOI] [PubMed] [Google Scholar]
- [32].Stokes DN, Hutton P. Rate-dependent induction phenomena with propofol: implications for the relative potency of intravenous anesthetics. Anesth Analg 1991;72:578–83. [DOI] [PubMed] [Google Scholar]
- [33].de Wit F, van Vliet AL, de Wilde RB, et al. The effect of propofol on haemodynamics: cardiac output, venous return, mean systemic filling pressure, and vascular resistances. Br J Anaesth 2016;116:784–9. [DOI] [PubMed] [Google Scholar]
- [34].Levitzky BE, Lopez R, Dumot JA, et al. Moderate sedation for elective upper endoscopy with balanced propofol versus fentanyl and midazolam alone: a randomized clinical trial. Endoscopy 2012;44:13–20. [DOI] [PubMed] [Google Scholar]
- [35].Aissaoui Y, Belyamani L, El Wali A, et al. Prevention of myoclonus after etomidate using a priming dose. Ann Fr Anesth Reanim 2006;25:1041–5. [DOI] [PubMed] [Google Scholar]
- [36].Sedighinejad A, Naderi Nabi B, Haghighi M, et al. Comparison of the effects of low-dose midazolam, magnesium sulfate, remifentanil and low-dose etomidate on prevention of etomidate-induced myoclonus in orthopedic surgeries. Anesth Pain Med 2016;6:e35333. [DOI] [PMC free article] [PubMed] [Google Scholar]


