Abstract
Rationale:
Paradoxical embolism (PDE) refers to direct passage of venous thrombi into the arterial circulation through an arteriovenous shunt.
Patient concerns:
Case 1 presented with initial symptoms of shock and cerebral infarction. Case 2 developed middle cerebral artery occlusion during angiography.
Diagnoses:
2 cases were diagnosed as PDE.
Interventions:
They received thrombolytic therapy and anticoagulant therapy.
Outcomes:
The patients had recovery.
Lessons:
This report highlights the myriad clinical manifestations of PDE and underlines the importance of meticulous history taking and physical examination for early diagnosis.
Keywords: atrial septal defect, deep vein thrombosis, paradoxical embolism, patent foramen ovale, pulmonary embolism
1. Introduction
Paradoxical embolism (PDE) refers to the direct passage of venous thrombi into the arterial circulation through an arteriovenous shunt. PDE is a rare phenomenon and accounts for <2% of all arterial emboli.[1] The diagnosis of PDE is often difficult owing to multisystemic involvement, and the condition is associated with a high fatality rate in the absence of specific treatment.[2] The clinical diagnostic criteria of PDE include evidence of venous or pulmonary embolism (PE), presence of intracardiac defect or pulmonary fistula associated with abnormal passage between the venous and arterial circulation, and evidence of systemic embolism.[3] Owing to its myriad clinical manifestations, the diagnosis of PDE requires a high index of suspicion.
In the present report, we present our experience with 2 cases of PDE. Key findings from a review of relevant literature are presented. This report was approved by the Ethics Committee at the First Affiliated Hospital of Xi’an Jiaotong University (China).
2. Case report
2.1. Case 1
An 82-year-old man was hospitalized due to sudden onset of chest pain. He complained of difficulty in breathing and cough since 1 week. Two days before admission, he developed pain and itching in the left leg, which was followed by slurring of speech, numbness in the left limbs, and difficulty in walking. He had a 5-year-long history of hypertension.
At admission, he developed syncope, hypotension, and tachycardia. His blood pressure (BP) was 60 to 90/40 to 50 mm Hg; heart rate (HR) was 100 to 200/min, and oxygen saturation of blood (SaO2) on persistent low flow oxygen therapy was 88%. Left upper limb had a low skin temperature and diminished radial pulse. Muscle strength in both left limbs was grade 4. On neurological examination, his tongue deviated to the left side on protrusion. There were no signs of deep vein thrombosis (DVT). Elevated level of D-dimer (16.5 mg/mL) was noted.
Transthoracic echocardiography (TTE) showed an atrial septal defect (ASD) complicated by atrial septal aneurysm (ASA) and a left to right shunt (Fig. 1A). Increased right ventricular systolic pressure (62.41 mm Hg) was suggestive of pulmonary arterial hypertension (PAH). Computed tomography (CT) of head showed multiple lacunar infarcts, cerebral white matter demyelination and encephalatrophy (Fig. 1B). Color Doppler flow imaging (CDFI) revealed total occlusion of middle-lower segment of the left brachial artery and its distal branches (Fig. 1C). Computed tomography pulmonary angiography (CTPA) showed extensive bilateral PE (Fig. 1D). Pulmonary atelectasis was found in the middle lobe of the right lung (Fig. 1E). DVT in the left lower limb and a filling defect in the right pulmonary artery were found on digital subtraction angiography (DSA) (Fig. 1F, G).
Figure 1.
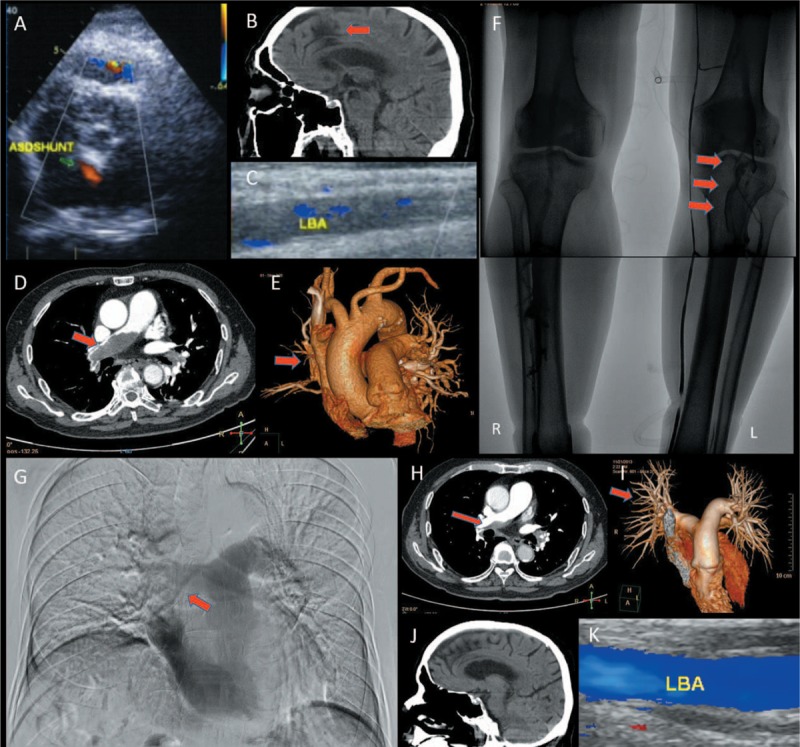
Imaging findings of case 1. (A) TTE showed an atrial septal defect complicated by atrial septal aneurysm, and a left to right shunt; (B) Head CT showed multiple lacunar infarcts, cerebral white matter demyelination, and encephalatrophy; (C) CDFI revealed total occlusion of middle-lower segment of the LBA; (D) CTPA showed extensive bilateral PE; (E) Pulmonary atelectasis was found in the middle lobe of the right lung; (F) Posterior tibial vein and anterior tibial vein were not visualized in ascending phlebography. The lower segment of peroneal vein and popliteal vein showed large filling defects (red arrow); (G) Pulmonary artery angiography showed a filling defect in the right pulmonary artery; (H, I) After 2 months of extramural hospital treatment, follow-up CTPA showed a significant improvement in the PE; (J) Head CT showing good recovery of cerebral lesions; (K) CDFI of the LBA showed good recovery.
His hemodynamic indices improved after administration of urokinase (250,000 units). Two hours after admission, his BP was 100/60 mm Hg, HR was 90/min, and SaO2 was >92%. When the patient's vital signs improved significantly (BP 144/69 mm Hg and HR 64/min), he was started on anticoagulation therapy with rivaroxaban (10 mg once daily). Decreased blood velocity was observed in inferior vena cava, and a Gunther tulip vena cava filter was placed.
The patient had an uneventful recovery and was discharged 13 days after admission on rivaroxaban. Beraprost sodium (20 μg twice daily) was prescribed to improve peripheral arterial circulation and to alleviate PAH. After 2 months of extramural hospital treatment, follow-up CTPA showed significant improvement in PE (Fig. 1H, I). TTE showed alleviation of PAH. Head CT showed resolution of lesions (Fig. 1J). On CDFI, a significant improvement in blood flow in the left brachial artery (Fig. 1K) and the left leg veins was observed.
2.2. Case 2
A 43-year-old woman presented with cyanosis and swelling of the fingers of the right hand. The right hand felt cool to touch. There was associated pain in the area of right deltoid muscle. One year before admission, she experienced swelling and pain of the right leg, which recovered spontaneously.
Physical examination showed decreased skin temperature and diminished radial pulse in the right upper extremity. She exhibited no signs of DVT. However, decreased levels of hematocrit (28.3%) and hemoglobin (80.0 g/L) were noted.
CDFI revealed occlusion of the right ulnar artery and decreased blood flow in the right subclavian artery (Fig. 2A). TTE showed a patent foramen ovale (PFO) with a left to right shunt (Fig. 2B). Angiography confirmed the occlusion of the right ulnar artery (Fig. 2C). The right posterior tibial and anterior tibial veins could not be visualized. The patient suddenly developed aphasia, urinary incontinence, and plegia of the left limbs during the angiography procedure. On neurological examination, her tongue deviated to the left side on protrusion, and was found to have mixed aphasia and a shallow nasolabial groove. Cerebral angiography revealed occlusion of the distal M1 segment of the right middle cerebral artery and the terminal part of the anterior cerebral artery (Fig. 2D). She was administered transcatheter thrombolytic therapy with urokinase (750,000 units). Twenty minutes later, the blood flow was restored (Fig. 2E). Subsequently, pulmonary artery angiography showed PE in the right middle lobe, which necessitated the placement of Gunther tulip vena cava filter. On the basis of magnetic resonance imaging (MRI) findings, a diagnosis of acute cerebral infarction (ACI) was established (Fig. 2F).
Figure 2.
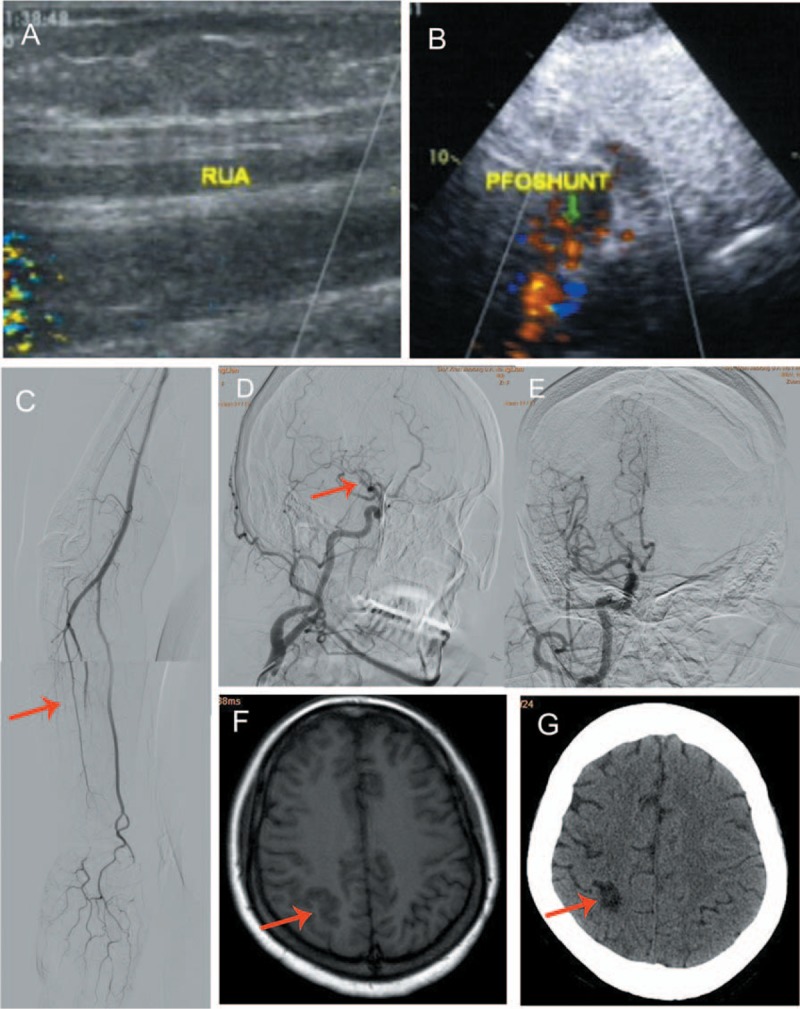
Imaging findings of case 2. (A) CDFI revealed an occlusive thrombus in the RUA; (B) TTE showed a patent foramen ovale with a left-to-right shunt; (C) Arteriography confirmed thromboembolic occlusion of the right ulnar artery; (D) Cerebral angiography showed occlusion of distal M1 segment of the right middle cerebral artery (red arrow indicates the site of interruption of blood flow); (E) Blood flow was restored 20 minutes after transcather thrombolytic therapy with urokinase; (F) MRI performed 24 hours later showed acute cerebral infarction; (G) Follow-up head CT showed small multiple foci of infarct and encephalomalacia of the right cerebrum after 2 months of anticoagulation therapy.
A diagnosis of upper extremity artery embolism, PFO, venous thromboembolism (VTE), and PE was made. Anti-platelet (aspirin, 100 mg, once daily) and anticoagulant therapy (warfarin, 2.5 mg, once daily) were started. Beraprost sodium was administered to improve peripheral artery circulation and to alleviate PAH.
Her symptoms improved gradually and she was discharged 13 days after admission on wafarin (3.125 mg, once daily). After 2 months of anticoagulation therapy, follow-up CT of head showed multiple small foci of infarct and encephalomalacia in the right cerebrum (Fig. 2G). CTPA showed regression of PE. CDFI of the arterial supply of the left upper extremity and venous circulation of the left leg showed significant improvement.
3. Discussion
PDE may have an insidious onset with mild symptomatology, which renders it liable to misdiagnosis. Prompt diagnosis is essential, as the condition is associated with potentially life-threatening complications. Meticulous history taking and physical examination at admission is crucial to recognize the less conspicuous signs. In case 1, it was easy to diagnose shock and ACI due to the typical clinical presentation, which included inability to use the left limb, syncope, hypotension, and tachycardia in the backdrop of hypertension. We initially performed electrocardiogram, TTE, and related laboratory investigations to determine the cause of shock. The discovery of PAH and the elevated D-dimer level were consistent with the diagnosis of PE. A combination of ACI and PE is common among the older people over the age of 80 years, and the diagnosis is usually straightforward. It is relatively easy to suspect PDE when a young person develops ACI. In older patients, the clinician may not suspect PDE, especially when hemodynamic restoration is a key priority. Low skin temperature of the left upper extremity and diminished radial pulse pointed toward acute limb ischemia (ALI). These signs are liable to be missed or disregarded owing to the apparent neuromuscular deficit. CDFI of the left upper limb artery confirmed the diagnosis of ALI. TTE revealed an ASD, due to which a diagnosis of PDE was highly suspected. Finally, DSA confirmed the diagnosis of PDE.
Case 2 sustained cerebral embolism during an interventional procedure. The origin of the embolus could not be easily determined initially. Our experience with this case underlines the importance of careful monitoring during interventional procedures. Such patients show remarkable recovery on timely institution of treatment.
Agarwal et al[4] reported occurrence of ALI and PE in a 41-year-old patient with hereditary spherocytosis. They suggested that in a patient with ALI and no obvious source of systemic arterial emboli, possibility of a right to left shunt should be considered. Although transesophageal echocardiography is a sensitive method, it may not be appropriate for critical patients like case 1, who had hemodynamic instability and hypoxemia. Catheter-directed thrombolytic therapy is recommended according to the Trans-Atlantic Inter-Society Consensus Document on Management of Peripheral Arterial Disease (TASC-II).[5] The venous vasculature should also be evaluated with DSA. It is noteworthy that both the patients in this report did not manifest typical symptomatology of DVT at admission.
The most effective medical therapy for secondary prevention of recurrent thromboembolic events is not known. Acetylsalicylic acid, oral anticoagulation, or a combination of both can be used. However, the optimal regimen that minimizes the risk of thrombotic events while avoiding bleeding complications is yet to be determined. In the case 2, the patient refused intervention for the closure of PFO. However, good results were achieved with oral anticoagulation in conjunction with retrievable IVCF[6,7] in both cases.
In conclusion, due to the myriad clinical manifestations of PDE coupled with potentially fatal complications, careful history taking and physical examination are essential to arrive at an accurate and rapid diagnosis.
Footnotes
Abbreviations: ALI = acute limb ischemia, ASA = atrial septal aneurysm, ASD = atrial septal defect, CDFI = color Doppler flow imaging, CT = computed tomography, CTPA = Computed tomography pulmonary angiography, DVT = deep vein thrombosis, HR = heart rate, PAH = pulmonary arterial hypertension, PDE = paradoxical embolism, PFO = patent foramen ovale, TTE = transthoracic echocardiography.
JG and HYT contributed equally to this work.
The authors report no conflicts of interest.
References
- [1].D’Audiffret A, Shenoy SS, Ricotta JJ, et al. The role of thrombolytic therapy in the management of paradoxical embolism. Cardiovasc Surg 1998;6:3302–6. [DOI] [PubMed] [Google Scholar]
- [2].Windecker S, Stortecky S, Meier B. Paradoxical embolism. J Am Coll Cardiol 2014;64:403–15. [DOI] [PubMed] [Google Scholar]
- [3].Johnson BI. Paradoxical embolism. J Clin Pathol 1951;4:316–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Agarwal SK, Binbrek AS, Thompson JA, et al. Massive pulmonary embolism and acute limb ischaemia in a patient of hereditary spherocytosis and patent foramen ovale. Heart Lung Circ 2010;19:742–4. [DOI] [PubMed] [Google Scholar]
- [5].Norgren L, Hiatt WR, Dormandy JA, et al. Inter-Society Consensus for the management of peripheral arterial disease (TASC II). J Vasc Surg 2007;45(Suppl):SS5–67. [DOI] [PubMed] [Google Scholar]
- [6].Wells PS, Forgie MA, Rodger MA. Treatment of venous thromboembolism. JAMA 2014;311:7717–28. [DOI] [PubMed] [Google Scholar]
- [7].Imberti D, Dentali F, Ageno W, et al. Evidence and clinical judgment: vena cava filters. Thromb Haemost 2014;111:4618–24. [DOI] [PubMed] [Google Scholar]


