Fig. 4.
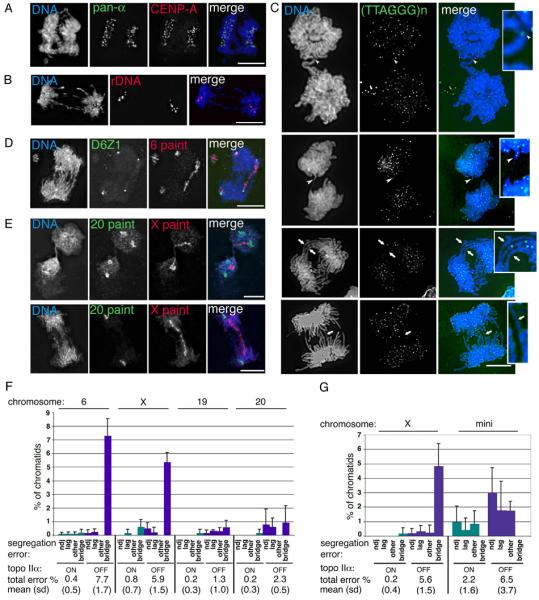
The effect of topo IIα depletion on human chromosome segregation. (A)-(E) HTETOP topo IIα-depleted (48 hours dox) segregating cells. (A) immuno-FISH showing co-localisation of segregating signals for the DNA pan-alphoid probe (green) and anti-CENP-A (red); (B) typical FISH image showing segregation of the rDNA loci (red); (C) four post-metaphase cells showing the segregation behaviour of telomeric regions, detected using a PNA anti-telomere probe (green). White arrowheads indicate telomeric DNA in chromatin bridges. White arrows point to chromatin bridges that lack detectable (TTAGGG)n signals. These regions have been enlarged for clarity. (D) in this anaphase the centromeres (green) of both copies of chromosome 6 have segregated, while the 6 paint (red) reveals that for one of these chromosomes the sister chromatids are bridged. This cell also appears to contain an acentric fragment of chromosome 6. (E) Two examples of segregating cells in which chromosome 20 (green) has separated correctly (although the second cell appears to have only one copy of this chromosome), while the sister chromatids of the single X chromosome (red) form a bridge. DNA is counterstained with DAPI – blue. Scale bar, 10 μm. (F) Chromosome paints and centromere-specific DNA probes were used to analyse the segregation of four of the endogenous HTETOP chromosomes: 6, the X, 19 and 20. For each chromosome the numbers of sister chromatids analysed from either untreated (topoIIαON) or 48 hours doxycycline-treated (topo IIαOFF) cells ranged from 141 to 447 per experiment. Shown are the means from 3 independent experiments (with s.d.). The total number of chromatids assessed for each chromosome under topo IIα ON/OFF conditions were: Chr. 6 - 1230 and 1183; Chr. X – 657 and 592; Chr. 19 – 1146 and 1058; Chr. 20 – 1298 and 1006. Segregation errors were classified as non-disjunction (ndj), lagging, bridging, or others (uncharacterised, apparent 1:0, segregation events, which might reflect absence of replication, chromosome loss or hybridisation failure). The combined segregation errors for each chromosome are summarised below the graph (mean percentage, s.d.). (G) The segregation behaviour of a 2.7 Mb human X centromere-based minichromosome was analysed following its transfer (by cell fusion) into HTETOP cells. Segregation data for the endogenous X chromosome in this hybrid background is also presented (mean and s.d.). For both the mini and X chromosomes the total number of sister chromatids examined from either untreated or 48 hours doxycycline-treated cells was ~1300 (based on data collected from 3 experiments for each of two independently-derived hybrid cell lines). The total levels of chromatid missegregation following topo IIα depletion are significantly higher, both for the whole X and for the minichromosome (Paired Student's t-Test, p ≤0.005).
