Figure 2. Tools to study membrane domain organization, composition and function.
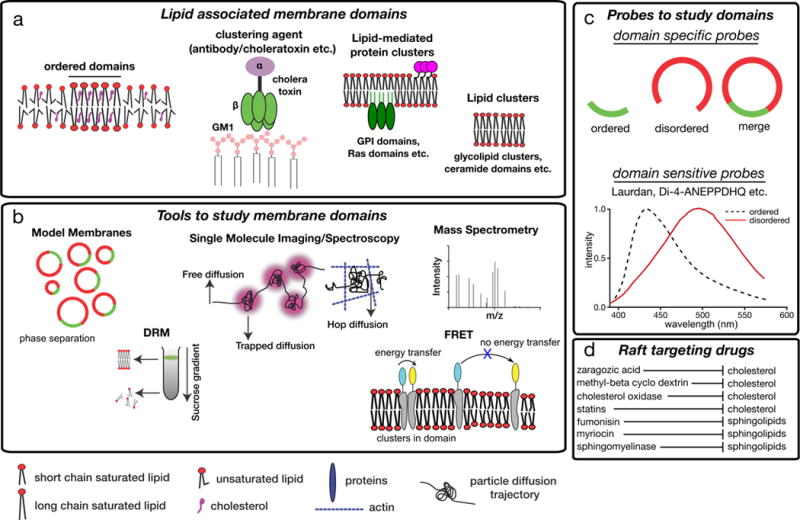
a | In principle, membrane domains can be pure lipid clusters, but in most physiologically-relevant cases, also involve proteins, including glycosylphosphatidylinositol (GPI)-anchored protein clusters or clusters of Ras proteins. These domains can be purely lipid-driven entities, such as domains established through liquid–liquid phase separation in model membranes. They can also be induced by clustering agents, such as cholera toxin which binds to monosialotetrahexosylganglioside (GM1) or by antibodies recognizing surface receptors b | Tools that are commonly used to investigate membrane domains. These include various model membranes (such as synthetic giant unilamellar vesicles (GUVs) and cell-derived giant plasma membrane vesicles (GPMVs)); detergent resistance assays, wherein raft-like membrane regions persist as detergent-resistant membranes (DRMs), whereas non-raft components are fully solubilized; single molecule imaging to evaluate the diffusion of membrane molecules; fluorescence spectroscopy (such as Förster resonance energy transfer (FRET)), and mass spectrometry. c | Also various probes can be used to study raft domains. Domain selective probes partition selectively to one of the domains while domain sensitive probes partition to both domains and change their photophysical behaviour (for instance absorbance and emission spectra) depending on the nature of the surrounding lipid environment. d | Treatments that interfere with cholesterol or sphingolipid levels have been used to disturb rafts in cells and can shed light on the cellular functions of these domains.
