Figure 4. Regulation of membrane domains.
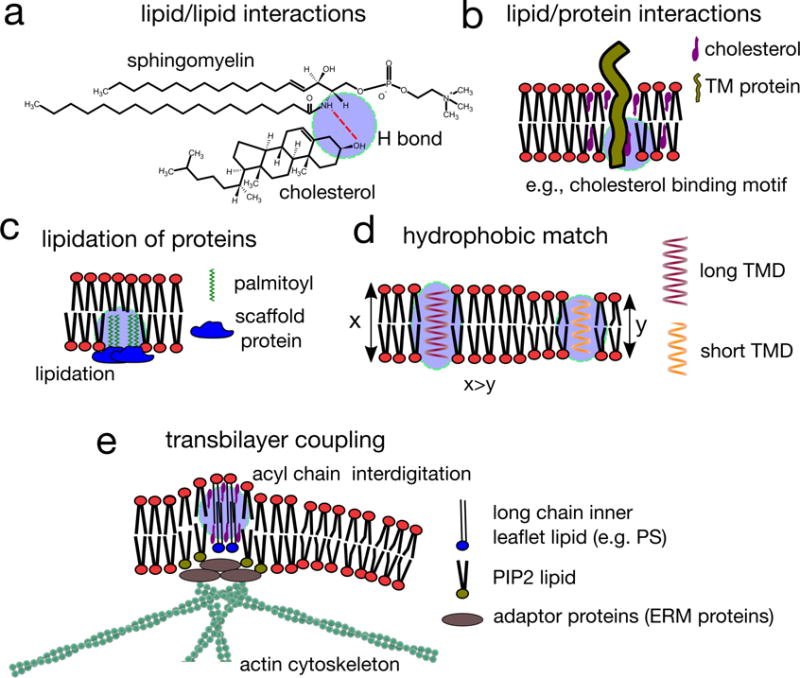
a | Lipid–lipid interactions, in particular interactions between cholesterol and sphingolipids (but also between other relatively saturated lipids) are the defining feature of lipid-driven ordered domain formation. The preferential interaction between sphingolipids and sterols is due to the saturation of sphingolipid hydrophobic tails, but also hydrogen bonding between these lipid species. The amide of the sphingolipid backbone can both donate and accept a hydrogen bond, and these hydrogen bonds are within the interfacial region of the membrane, where the relative paucity of water increases the relative stability of these bonds. b | Some proteins harbour lipid binding domains, interacting with cholesterol or sphingolipids, and these lipid–protein interactions may determine the affinity of proteins for ordered lipid domains. c | Lipidated proteins, modified by the attachment of a saturated acyl chain (such as palmitoyl moieties), are recruited to raft domains, but may also nucleate and recruit membrane domains if they are intergrated into a relatively static protein scaffold. d | Hydrophobic interactions can contribute to membrane domain organization and composition. In particular, proteins possessing transmembrane domains (TMD) of different lengths prefer different lipid environments to protect their hydrophobic TMDs from exposure to the aqueous surrounding. For example, proteins with long TMDs were found to associate with domains harbouring long chain saturated lipids (top). When there is a mismatch between the length of the TMD and the local lipid environment in which the protein resides, protein–protein interaction might be favoured instead, leading to local protein concentration (bottom). e | The immobilization of inner leaflet lipids containing long saturated acyl chains by actin clusters results in the engagement of long acyl chain containing lipid-anchored proteins (such as glycosylphosphatidylinositol (GPI)-anchored proteins) located in the outer leaflet, which, in the presence of cholesterol, induces their active clustering. This results in locally ordered, transbilayer nanodomains, which are dynamic owing to the dynamics of actin clusters, and may form even in conditions that do not favour liquid–liquid phase separation of lipids or other supporting interactions.
