Abstract
Social relationships are crucially dependent on individual ability to learn and remember ecologically relevant cues. However, the way animals recognize cues before engaging in any social interaction and how their response is regulated by brain neuromodulators remains unclear. We examined the putative involvement of arginine vasotocin (AVT) and isotocin (IT), acting at different brain regions, during fish decision-making in the context of cooperation, by trying to identify how fish distinguish and recognize the value of other social partners or species. We hypothesized that the behavioural responses of cleaner fish clients to different social contexts would be underlain by changes in brain AVT and IT levels. We have found that changes in AVT at the level of forebrain and optic tectum are linked with a response to allopatric cleaners (novel or unfamiliar stimuli) while those at cerebellum are associated with the willingness to be cleaned (in response to sympatric cleaners). On the other hand, higher brain IT levels that were solely found in the diencephalon, also in response to allopatric cleaners. Our results are the first to implicate these nonapeptides, AVT in particular, in the assessment of social cues which enable fish to engage in mutualistic activities.
Introduction
Social relationships are crucially dependent on individual ability to learn and remember beneficial cues [1]. The ability to pay attention to specific key information linked to other individuals or species is a prerequisite for animals that live in complex social systems. For instance, cues indicating that an animal is being watched, i.e., has an audience, lead to increases in levels of cooperation [2,3]. Social discrimination acquires a new dimension when occurring in cooperative or mutualistic contexts, as individuals’ decisions will determine the amount of benefits they gain but also depend on the responses of other individuals [4–6]. Typically, the term cooperation refers to a relationship between relatives in which both may asymmetrically benefit from the association [7,8], while the term mutualism is more specific to a similar cooperative relationship but this time, occurring between two different species [9].
Before engaging in any social interaction, animals need to assess the situation either by recognition of signalling cues associated with deceptive partners (e.g. individuals with mimicry strategies that exhibit specific shapes, colours or stripes [10]) or recollection of past interactions with less cooperative partners (e.g. partner that did not reciprocate equally in the past [11]).
Understanding how animals use new and previously acquired information to make decisions is particularly important to gain a clear view on: i) what makes individuals more, or less predisposed to cooperate, ii) how they respond to mixed cue signals (e.g. contradictory signals) that may differ in value and iii) how their responses are linked with brain neuromodulators. Among signalling neuromodulators are mammalian nonapeptides arginine vasopressin (AVP) and oxytocin (OT) and their teleostean homologs, arginine vasotocin (AVT) and isotocin (IT). They belong to an ancient family of neurohormones with an evolutionarily conserved structure and core physiological and behavioural functions across vertebrate taxa, particularly those related to regulation of vertebrate social behaviour [12,13,14]. In fish, the AVT/IT neurosecretory system contains three main cell groups distributed in the preoptic area (POA): gigantocellular, magnocellular and parvocellular, that project fibers to multiple target areas, such as ventral telencephalon, diencephalon, and various mesencephalic structures, in addition to projections to the neurohypophysis [15]. There is much evidence in fish that these neurohormones are implicated in aggressive behaviour [16,17], the promotion of territorial behaviour [18,19], changes in courtship behaviour [20,21], social status [22], pair formation [23], paternal care [24] and mutualistic behaviour [25–29].
One of the most well-known examples of cooperation between different species is the case of the interactions between cleaner fish and their client fishes. Cleaner fishes are specialized in inspecting the body surface, gill chambers and mouth of cooperating larger fishes, which are known as clients, in search of ectoparasites, mucus and dead or diseased tissues [30–34]. These cleaners must interact with a myriad of visiting species (dangerous piscivores to harmless herbivores) that may harbour more or less parasites and/or mucus, hence varying in value [35]. It is the quality of cleaners’ service that will determine the frequency of clients’ visits to their territories [see 36]. Regarding the best-known species of Indo-Pacific cleaner fish, Labroides dimidiatus there is emerging evidence pointing towards a crucial role of AVT as a modulator of their cleaning activities: intramuscular administration of AVT reduced their propensity to engage in interspecific cleaning activities and increased their motivation to engage with conspecific partners [25]. More recently, Cardoso and colleagues found that intramuscular AVT reduced a dimension of cooperativeness of individuals [26] and mediated associative learning abilities of cleaners depending on tasks (cue vs place discrimination) [27].
On the other side of these fish mutualisms, the clients, which regularly visit cleaners at their territories, face a different challenge: they must choose to visit amongst potential cleaner fish partners that may cooperate (e.g. that mostly forage on client’s ectoparasites), or not (e.g. cleaners that also feed on clients’ mucus which is detrimental to clients’ organism; for review see [36]). During their early life stages clients need to learn to seek, recognize and interact with several cleaners, identifying signaling cues such as their specific colors, stripes or shape to get the best service, i.e. removing the ectoparasites or gaining physical stimulation. Recent research has demonstrated that clients that interact more frequently with cleaners have better body condition [37] and the recurrent physical contacts contribute to reducing their stress levels [38]. The level of benefit arising from these interactions depends on ability of client fish to identify the specific cues to safely approach the fish providing an honest, fair cleaning service and avoid dubious cues, such as those coming from “false” or mimic cleaners. The false cleaners copy both appearance and behaviour of obligatory cleaners as L. dimidiatus but instead of cleaning they bite their clients [39]. However, the mechanisms involved in the regulation of such behaviour has not yet been determined, particularly those that help naïve coral reef fishes to learn and remember novel information, which they can use in future interactions.
There are only few studies that measured AVT and IT concentrations in different regions of fish brain in order to link them with the expression of different social behaviours [see 40,41], and only two that focus specifically on mutualistic behaviour [28,29]. In our previous study on AVT levels in different brain regions in four species of Labrid fish, we found that in the cerebellum of the obligate cleaners L. dimidiatus and Labroides bicolor, the levels were higher than those in facultative cleaner species and a non-cleaner species [29]. We suggested that AVT levels in the cerebellum can be associated with the expression of mutualistic behaviour. Furthermore, higher levels of AVT in the whole brain and forebrain of the obligate L. bicolor were associated with an increase of aggressiveness towards clients and roaming behaviour [29]. In another study, Cardoso and colleagues identified a link between brain isotocin levels and the quality of relationship within mixed sex couples of cleaners, i.e. forebrain IT levels were higher in those males that received more tactile stimulation from female partners [28]. Therefore, further research on the distribution of AVT and IT across different brain areas, in which they are hypothesized to act, is a promising approach.
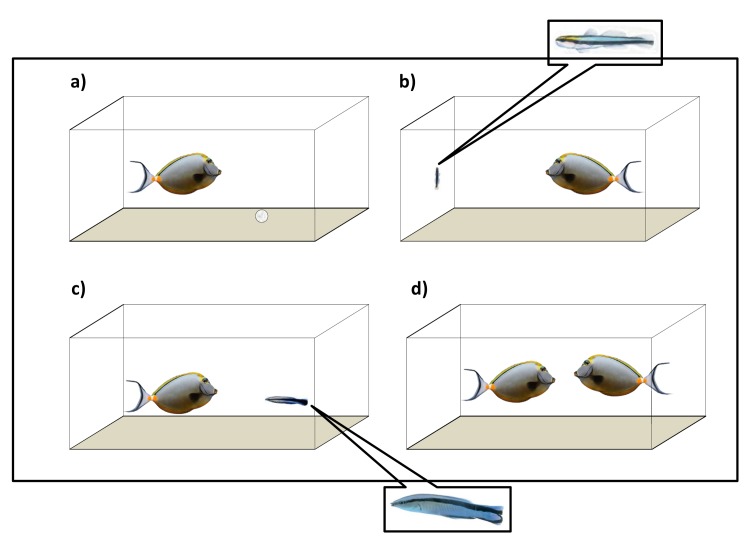
Here, we investigate if different signals are translated into changes in behaviour and brain nonapeptide’ levels in a coral reef fish, the Indo-Pacific blonde naso tang Naso elegans (family Acanthuridae). Naso tang is a potentially frequent client of the cleaner wrasse L. dimidiatus (both species originate from the Indo-Pacific region) and can be easily adapted to laboratory conditions. The aim of the present study is to examine the role of AVT and IT, acting at different brain regions, in client behavioural responses to cleaners. In laboratory experiments, we introduced wild-caught clients (Indo-Pacific N. elegans) to different social partners (experimental treatments) using a sympatric or recognizable (Indo-Pacific conspecifics and heterospecifics) and allopatric or non-recognizable (Atlantic originated heterospecifics, see Fig 1) cleaners. We assumed that the wild-caught clients encountered sympatric cleaner species before in their habitat as they are ubiquitous and occupy the same areas. We hypothesized that clients’ behavioural response to different social partners (experimental treatments) would be associated with changes in brain AVT and IT levels.
Fig 1. Experimental setup.
(A) Individual client fish (Indo-Pacific Naso elegans) is introduced to a novel object (a white ball), (B) Individual client fish is introduced to an allopatric cleaner species (the Caribbean cleaning goby Elacatinus evelynae), (C) Individual client fish is introduced to a sympatric cleaner species (the Indo-Pacific cleaner wrasse Labroides dimidiatus) and (D) Individual client fish is introduced to a conspecific. Images of fish are not in real scale, particularly those of the cleaner fish L. dimidiatus and E. evelynae when compared with client N. elegans size.
Materials and methods
Ethical note
The protocols were carried out in accordance to the approved guidelines by the Oceanário de Lisboa (fish housing facilities), where the experiments were then developed. Animal procedures used in this study were also approved by the Portuguese Veterinary Office (Direcção Geral de Veterinária, license # 0420/000/000/2009).
Animals and housing
Experiments were conducted at the fish housing facilities of the Oceanário de Lisboa (Lisbon, Portugal). The specimens used in this study were adult blonde naso tang Naso elegans (family Acanthuridae, also known as clients), the Indo-Pacific bluestreak cleaner wrasse Labroides dimidiatus and the Caribbean sharknose cleaning goby Elacatinus evelynae), all imported to Portugal by a local distributor (Tropical Marine Centre, Lisbon, Portugal). Total length (TL) and total weight (TW) of tang N. elegans ranged from 6.9 to 15.5 cm (mean ± SE: 10.41 ± 0.34 cm) and 4.9 to 78.1 g (19.66 ± 2.15 g). Tangs were kept in stock aquaria of 100x40x40 cm and cleaning gobies in aquaria of 50x40x40 cm, in groups of 5 to 10 individuals, while cleaner wrasses were kept alone in 50x40x40 cm aquaria. All aquaria were combined in a flow through system that pumped water from a larger sump (150x50x40 cm) that served as a mechanical and biological filter. Nitrite concentration was kept to very low levels (always below 0.3 mg/L). Each tank contained an air supply and a commercial aquarium heater (125W, Eheim, Jäger). PVC pipes (15–20 cm long; 20 cm diameter) served as shelter for the fish. Experiments were carried out between September and November 2012 in the individual smaller tanks (50x40x40 cm).
Experimental design and sampling
As mentioned previously, because the clients N. elegans and the cleaner wrasse L. dimidiatus both originated from Maldives (Indian Ocean), the latter were classified as sympatric cleaners in contrast to the Caribbean (Atlantic Ocean/Caribbean) originated cleaning gobies classified as allopatric cleaners. On each test day, the following experimental treatment groups were randomly allocated to focal clients (subjects): a) ball (a heavy, white ball, about 5 cm in diameter, permanently attached to the bottom), b) sympatric cleaner (L. dimidiatus), c) allopatric cleaner (E. evelynae) and d) conspecific (N. elegans), see Fig 1. Clients were distributed across experimental treatments as follows: 11 individuals for the sympatric cleaner group, 8 for the allopatric cleaner group, 10 for the conspecific group and 11 for the ball group. Each client was introduced into the experimental tank and left for at least 2–5 min until regular activity was restored (i.e. individuals were swimming normally). Experimental aquaria were also divided by opaque partitions preventing any visual contact between fish during experiments. Behaviour was then videotaped for the next 60 minutes while the experimenter left the room (see behavioural analyses section below). At the end of experiments, each tang was rapidly captured and sacrificed with an overdose of tricaine solution, a powerful anesthetic (MS222, Pharmaq; 500–1000 mg/L) and the spinal cord sectioned (both methods aimed to reduce fish suffering). The brain was immediately dissected under a stereoscope (Zeiss; Stemi 2000) into five macro-areas: forebrain (olfactory bulbs + telencephalon), diencephalon, optic tectum, cerebellum and brain stem, by separating along the natural boundaries between macro-areas, using the same methodology applied by [40,41,42] (see the dissection procedure at S1 File). Major brain areas were weighed and stored at—80°C.
Quantification of nonapeptides by high performance liquid chromatography with fluorescence detection (HPLC-FL)
Brain samples weights were used for later calculation of nonapeptide’ levels (peptide content was expressed per milligram of brain sample). Then, they were sonicated in 1 mL Milli-Q water (MicrosonXL, Misonix, USA), acidified with glacial acetic acid (3 μL) and placed in a boiling water bath for 3.5 min. Next, homogenates were centrifuged (12,000 g, 20 min, 4°C) and supernatants decanted and loaded onto previously conditioned (1 mL methanol, 1 mL Milli-Q water) solid phase extraction (SPE) columns (30 mg/1 mL, Strata-X, Phenomenex). To purify samples, columns were washed successively with 0.6 mL Milli-Q water and 0.6 of mL of 0.1% TFA (trifluoroacetic acid) in 5% acetonitrile. The peptides were eluted using 1.2 mL of 80% acetonitrile. The eluate was evaporated to dryness using a Turbo Vap LV Evaporator (Caliper Life Scence, USA). Samples were then frozen and stored at -80°C prior to HPLC analysis.
Before quantitative analysis, the samples were re-dissolved in 40 μL of 0.1% TFA in 30% acetonitrile and divided into two for replication. Pre-column derivatization of AVT and IT was performed according to the procedure by Gozdowska and colleagues [43]. For derivatization reaction, 20 μL of sample and 20 μL of 0.2 M phosphate buffer (pH 9) were mixed, and then 3 μL of NBD-F (4-fluoro-7-nitro-2,1,3-benzoxadiazole: 30 mg in 1 mL of acetonitrile) was added. The solution was heated at 60°C for 3 min, cooled on ice, acidified with 4 μL of 1 M HCl and eluted in a HPLC column. Derivatized samples were measured with Agilent 1200 Series Quaternary HPLC System (Agilent Technologies, USA). Chromatographic separation was achieved on an Agilent ZORBAX Eclipse XDB-C18 column (150 mm x 4.6 mm I.D., 5 μm particle size). The gradient elution system was applied for separation of derivatized peptides. The mobile phase consisted of solvent A (0.1% TFA in H2O) and solvent B (0.1% TFA in acetonitrile: H2O (3:1). A linear gradient was 40–65% of eluent B in 20 min. Flow rate was set at 1 mL/min and the column temperature set to 20 oC. Injection volume was 47 μL. Fluorescence detection was carried out at 530 nm with excitation at 470 nm (calibration data and curves are provided at Table A and Fig A in S1 File).
Behavioural analyses
During each video analysis, we recorded for the focal client N. elegans the following measures, during the 60 minutes of observation: 1) the number and duration (in seconds) of each cleaning inspection received; 2) the frequency and duration of tactile stimulation received (where a cleaner touches, with ventral body and fins, the body of the client and no feeding is involved [44]); 3) the number of jolts by clients (cleaners sometimes bite clients and they respond with a short body jolt which usually is a behaviour associated with cheating by cleaner fish [45,46]); 4) number and duration of chases that occurred when the focal individual rapidly advanced toward the other (either a cleaner or conspecific, in seconds) and finally 5) number of bites provided by focal individual. Moreover, it is important to note that the context in which these chases occur (either against sympatric cleaners or against other conspecifics) may be quite distinct. In the wild, this usually happens that clients chase cleaners, because cleaners were aggressive towards the clients or clients jolted previously [47]. In the conspecific context, the incidences of chases by the subject may be due to size differences (if intruder is larger than the resident) or to sex differences. Although we tried to match the sizes of the individuals, this was not always possible.
Statistical analyses
A total of 40 clients were used for brain AVT and IT measurement. Of these, according to E2 measurements (see S1 File for protocol), 18 were males (mean ± SD: 0.0045 ± 0.0022 ng/mg) and 22 were females (0.057 ± 0.073 ng/mg). In four individuals, AVT levels were below the limit of detection. Brain IT and AVT levels (brain and behavioural variables) were log transformed to conform to parametric parameters of homogeneity of variances (assessed by Levene’s test). In each experimental context the following behavioural measures were calculated: a) the frequency of cleaning interactions (per 60 min), b) mean inspection duration (in seconds), c) proportion of interactions in which tactile stimulation was applied to clients (number of interactions in which tactile stimulation was provided/ total number of interactions), d) frequency of jolts per 100s of inspection, e) frequency of antagonistic charges (chases per 60 min), f) duration of chases (in seconds) and g) frequency of bites given by focal individual (per 60 minutes). Brain levels of AVT and IT for the whole brain and each brain macro-area (forebrain, diencephalon, optic tectum, cerebellum and brain stem) were first compared by using two way independent measures, with sex and treatment as fixed factors. Because sex was not found to be a significant factor, it was the dropped from all models: forebrain (AVT): F(3,35) = 0.191, p = 0.902; forebrain (IT): F(3,36) = 1.796, p = 0.168; diencephalon (AVT): F(3,35) = 0.126, p = 0.944; diencephalon (IT): F(3,36) = 0.774, p = 0.517; optic tectum (AVT): F(3,35) = 1.051, p = 0.384; optic tectum (IT): F(3,36) = 1.816, p = 0.160; cerebellum (AVT): F(3,35) = 0.653, p = 0.588; cerebellum (IT): F(3,36) = 0.279, p = 0.840; brain stem (AVT): F(3,35) = 0.866, p = 0.156; brain stem (IT): F(3,36) = 0.109, p = 0.955). One way ANOVAs, followed by Tukey post-hoc HSD tests tested for the effect of treatment on AVT and IT brain levels. Relationships within and between behavioural measures and clients’ brain neuropeptide levels were examined by using the Pearson correlation coefficients. The Hochberg-adjusted p-values [48], which provide adjusted alpha values to account for multiple comparisons, were calculated in order to account for multiple comparisons. Therefore, we report the exact p-value produced for each correlation analysis and indicate whether it fell below its adjusted alpha. All tests were two tailed, the ANOVAs were performed with the R software [49] and Pearson correlations were performed in the software package SPSS Statistics, version 22.
Results
Client behaviour
There were differences in the behavioural response of client Naso tang (hereafter referred to as clients) across experimental treatments (Fig 1). Behavioural interactions occurred in two experimental treatments: clients interacted with the sympatric cleaner fish (L. dimidiatus) as well as with conspecifics (see Table 1). Clients that were cleaned more frequently by sympatric cleaners appeared to receive less tactile stimulation (Pearson correlation test: r = - 0.64, N = 10, P = 0.04), were observed to jolt more frequently (r = 0.66, N = 10, P = 0.04) and also chased cleaners more often (r = 0.72, N = 10, P = 0.02). The frequency of jolts during inspection was significantly correlated with the frequency of chases (r = 0.88, N = 10, P = 0.001). When introduced to an allopatric cleaner species (cleaning gobies), clients avoided contact with gobies and did not approach the gobies at all. Clients did not approach or have any contact with the ball either (see Table 1).
Table 1. Frequency of observed behavioural measures for each experimental treatment.
These include: a) the frequency of cleaning interactions, b) mean inspection duration, c) proportion of interactions in which tactile stimulation was applied to clients, d) frequency of jolts per 100 s of inspection, e) frequency of chases, f) duration of chases and g) frequency of bites given by focal individual. Mean ± Standard Error (SEM) are provided for each behavioural measure.
|
Behaviour |
Experimental treatments | |||
|---|---|---|---|---|
| Sympatric cleaner | Allopatric cleaner | Conspecific | Ball | |
| Frequency of cleaning interactions | 14.00 ± 2.75 | 0 | 0 | 0 |
| Inspection duration (in sec) | 7.33 ± 1.22 | 0 | 0 | 0 |
| Proportion of interactions with tactile stimulation | 0.17 ± 0.04 | 0 | 0 | 0 |
| Proportion of time providing tactile stimulation | 0.27 ± 0.06 | 0 | 0 | 0 |
| Jolts (per 100s of inspection) | 0.42 ± 0.22 | 0 | 0 | 0 |
| Frequency of chases (by focal) | 0.90± 0.54 | 0 | 6.00 ± 2.85 | 0 |
| Chase duration (in sec) | 1.20± 0.51 | 0 | 22.00 ± 10. 44 | 0 |
| Frequency of bites (provided by focal) | 0 | 0 | 0.45 ± 0.33 | 0 |
Client brain neuropeptides
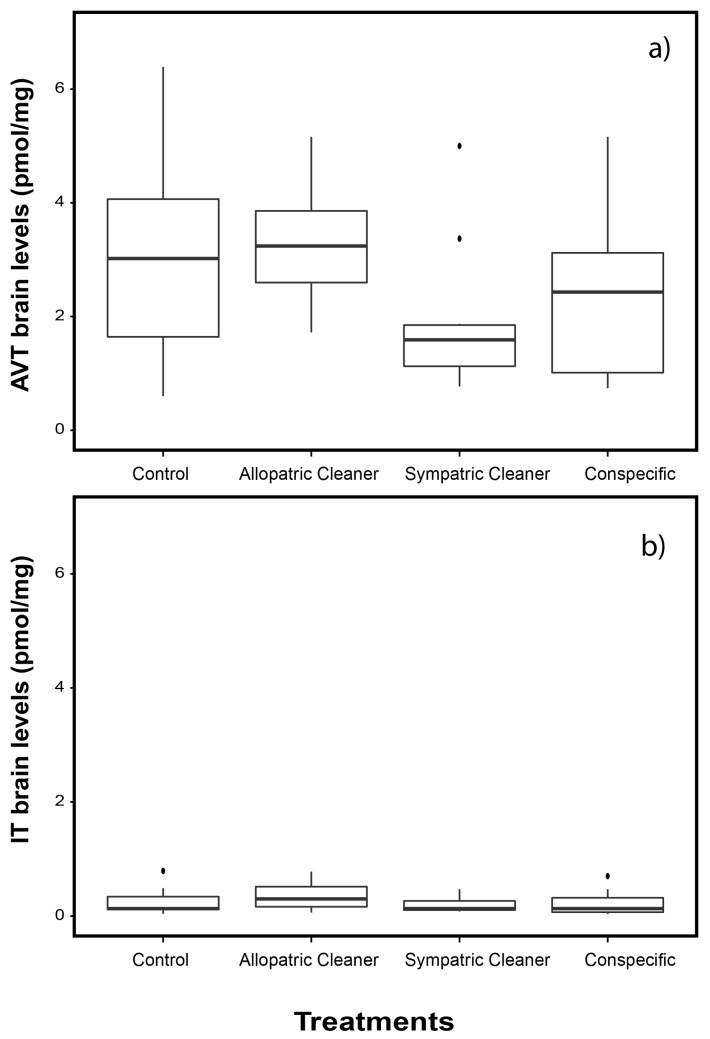
Mean concentrations of AVT and IT in the whole brain and brain macro-areas are given in Figs 2, 3 and 4 and Table B in S1 File. No differences were found in whole brain AVT levels across experimental treatments (one way ANOVA, F3,36 = 2.183, p = 0.107, Fig 2A).
Fig 2. Whole brain nonapeptide’ levels in clients (Naso elegans).
(A) levels of arginine vasotocin (AVT) and (B) levels of isotocin (IT) in four treatment groups: a) sympatric cleaner (Labroides dimidiatus), b) allopatric cleaner (Elacatinus evelynae), c) conspecific (N. elegans) and d) ball, expressed as AVT (pmol/mg) and IT (pmol/mg). Medians (full lines) and interquartile range are presented in boxes.
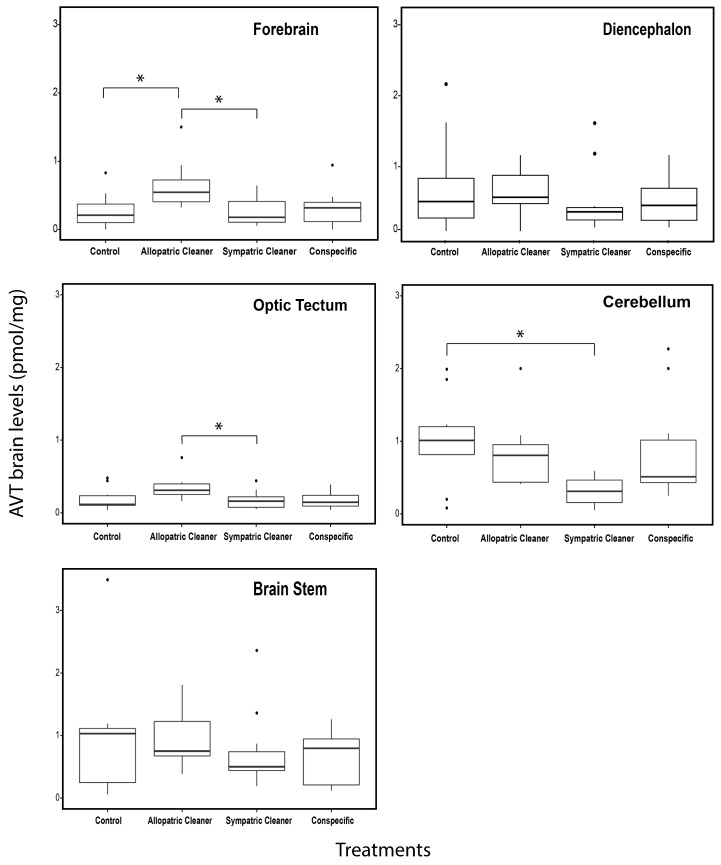
Fig 3. Levels of arginine vasotocin (AVT) in different brain macro-areas: Forebrain, diencephalon, optic tectum, cerebellum and brain stem in clients (Naso elegans).
Four treatment groups: a) sympatric cleaner (Labroides dimidiatus), b) allopatric cleaner (Elacatinus evelynae), c) conspecific (N. elegans) and d) ball, expressed as AVT (pmol/mg). Medians (full lines) and interquartile range are presented in boxes.
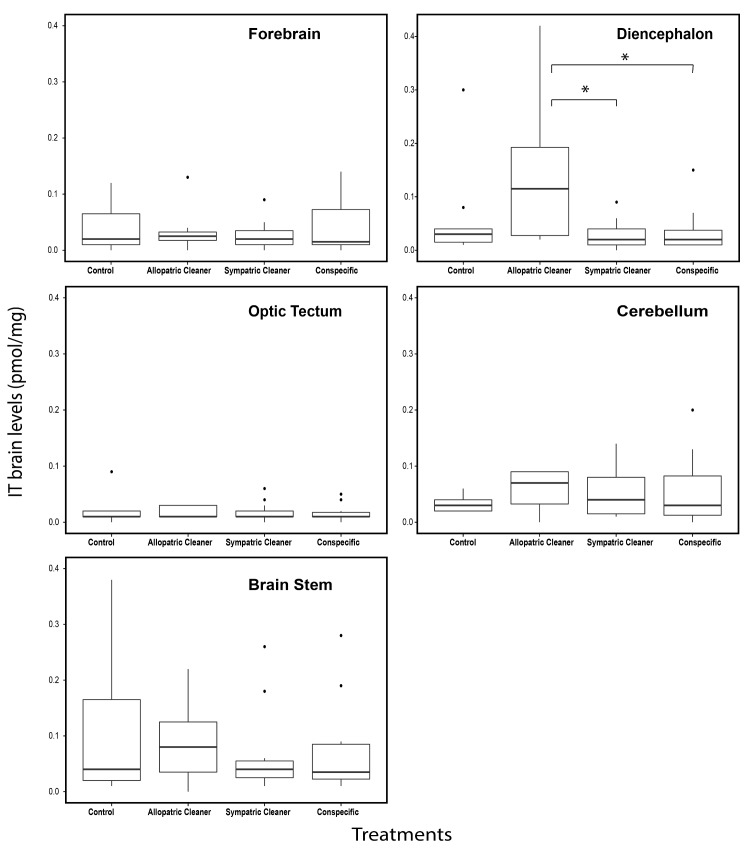
Fig 4. Levels of isotocin (IT) in different brain macro-areas: Forebrain, diencephalon, optic tectum, cerebellum and brain stem in clients (Naso elegans).
Four treatment groups: a) sympatric cleaner (Labroides dimidiatus), b) allopatric cleaner (Elacatinus evelynae), c) conspecific (N. elegans) and d) ball, expressed as IT (pmol/mg). Medians (full lines) and interquartile range are presented in boxes.
Brain AVT levels differed significantly at the forebrain, optic tectum and cerebellum across experimental treatments (forebrain: F3,35 = 3.282, p = 0.032; optic tectum: F3,35 = 3.274, p = 0.032 and cerebellum: F3,35 = 4.822, p = 0.006, see Fig 3, Table B in S1 File) but not at the diencephalon or brain stem (diencephalon: F3,35 = 0.365, p = 0.779 and brain stem: F3,35 = 0.628, p = 0.602, see Fig 3, Table B in the S1 File). Higher brain AVT levels were found at the forebrain and optic tectum of clients in contact with allopatric cleaners when compared with those introduced to sympatric cleaners (forebrain: p = 0.03; optic tectum: p = 0.04, see Fig 3, Table C in S1 File); and at the forebrain of those in contact with allopatric cleaners compared to those with a ball (forebrain: p = 0.035, Fig 3, Table C in S1 File). Clients in contact with sympatric cleaners showed significantly lower levels of brain AVT in the cerebellum, when compared to those introduced to a ball (p = 0.01, Fig 3, Table C in S1 File), while no significant differences were found when clients were in direct contact with conspecifics and allopatric cleaners (client introduced to sympatric cleaner vs conspecific p = 0.07; client introduced to sympatric vs allopatric cleaner p = 0.07, Fig 3, Table C in S1 File).
No differences were found in whole brain IT levels across experimental treatments (one way ANOVA, F3,36 = 0.896, p = 0.452, Fig 2B). Brain IT level differed significantly only at the diencephalon (diencephalon: F3,36 = 3.8, p = 0.02; Tables B and D in S1 File, for results in other brain macro-areas, Fig 4). Clients in contact with allopatric cleaners had higher brain IT level at the diencephalon compared with those introduced to sympatric cleaners or conspecifics (clients introduced to allopatric vs sympatric cleaners, p = 0.03; and allopatric cleaners’ vs conspecifics, p = 0.03, see Table D in S1 File, Fig 4).
Regarding the relationship between client behaviour and brain neuropeptide, none of the correlations remained significant after calculation of Hochberg-adjusted p-values [48] (Tables E and F in S1 File).
Discussion
In nature, individuals must navigate complex social environments. Here, we introduced clients to treatments that differed substantially: a) the conspecific context was both familiar and social, b) the sympatric cleaner condition was familiar, cooperative, and social, c) the allopatric cleaner condition was novel, social, and potentially unsafe and d) the control condition was non-social and novel. Specifically, we showed that clients i) engaged mostly in agonistic interactions with conspecifics, primarily on cleaning interactions with sympatric cleaners, ii) did not engage in agonist or cleaning interactions with allopatric cleaners and iii) were similarly interactively absent in the control context (see Fig 1 for experimental setup); we found that both nonapeptide levels (AVT and IT) differed overall across some specific brain regions, changing in accordance to specific treatment. We found that clients in contact with allopatric cleaners had higher levels of AVT in the forebrain and the optic tectum than those introduced to sympatric cleaners. On the other hand, clients in contact with sympatric cleaners showed lower levels of AVT in the cerebellum compared to those kept with a ball. Moreover, levels of IT were higher in subjects in contact with allopatric cleaners when compared to sympatric cleaners and conspecifics only at the diencephalon. Our results are the first to link these nonapeptides with the variation of client social behaviour and probability to engage in cooperative interactions in fish.
Clients in contact with never-before seen cleaning gobies, a species of Atlantic (Caribbean) origin, shared a common behavioural response: they kept at a distance and never interacted. Some may have ventured and explored, e.g. got to observe the gobies a bit closer, but only temporarily. Nonetheless, clients seemed to be extremely aware of goby’ presence (for instance, by moving when gobies moved sharply in their direction), perhaps in trying to determine the potential risk associated to these cleaning gobies. These cleaning gobies share common features with other cleaner fish species, such as contrasting colorful stripes, which they use to advertise their cleaning services [50,51]. For example, the Caribbean cleaning gobies E. evelynae (used in this study) also display specific phenotypic features such as blue and yellow stripes, which normally signal cleaning service for coral reef fish [51] and evokes clients’ curiosity. However, the display of conspicuous color stripes is also used by other species to signal other forms of communication, which is the case of some cleaner fish mimics [52,53]. Considering the lack of response by clients introduced to a novel object (ball), especially when compared with those introduced to a novel cleanerfish species (gobies), novelty was probably not the sole factor influencing these animals’ response to gobies.
We found that subject clients, introduced to an allopatric cleaner, had higher concentrations of forebrain AVT levels compared to those in contact with a sympatric cleaner fish species and those presented to a static white ball. A similar result was observed at the optic tectum (an area of visual integration), with clients having higher AVT levels when in contact with allopatric cleaners compared to sympatric cleaners. In teleost fish, the forebrain, namely the dorsomedial (Dm) telencephalon, a partial homolog region of the mammalian amygdala, is known to be involved in fear based conditioning (learning to avoid noxious or harmful stimuli) [54–57]. Mapping of AVT receptors in the brain of teleost species have shown that V1a receptors are widely distributed throughout the forebrain, being specifically present on the Dm region but also at optic tectum [58–61]. Thus, the increase of AVT levels in both the optic tectum and the forebrain regions of those in contact with allopatric cleaners (compared to sympatric cleaners and control) provides an indication that AVT is conveyed to these areas and may be involved in the perception of novel and potential noxious /unsafe stimuli.
Clients in contact with a sympatric cleaner species had lower AVT levels at the cerebellum when compared with those introduced to a ball (control). Interestingly, when looking at the initial correlation trends, before correcting the p values, there seemed to be a negative link between the duration of clients’ interaction with native cleaners and a reduction of AVT levels in the cerebellum. Hence, it appears that one of the most likely reasons for AVT decrease at the cerebellum is the length of interactions with cleaners. These results are in line with previous studies by Soares and colleagues, done in the wild, in which exogenously infused AVT decreased the likelihood of engaging of native cleaners (L. dimidiatus) in cleaning interactions with their clients [25]. Thus, lower levels of AVT at the cerebellum may be linked to a higher propensity to interact with cleaners. Furthermore, the cerebellum of teleost fish, which is traditionally associated with motor control, is also highly implicated in several cognitive and emotional functions, particularly in classical conditioned /associative learning and memory processes [62,63]. Indeed, it is through classical associative learning that clients first acquire the iterated training that makes them visit these cleaners’ territories, by associating the interaction with a cleaner with benefits, such as: lowering parasite levels, gaining tactile stimulation, or cortisol levels reduction [64]. Thus, there is a potential higher scope for the influence of AVT on cleaners’ learning abilities happening at the cerebellum level but most likely in straight connection with other important brain areas, such as the telencephalon. However, because we do not have any direct proof that each of the subjects used had already been in contact with cleaners (before experimental treatments), we cannot be sure that the effect observed in AVT levels is due to learned memory/recognition or to genetically-inherited predisposition to interact with cleaner species. In future studies would be interesting to include a previous manipulation of individual subject familiarity in relation to each cleaner and/or conspecific introduced.
In the present study, higher values of IT were found solely at the diencephalon, in subject clients put in contact with allopatric cleaning gobies compared to those interacting with sympatric cleaner fish species or conspecifics. Recent studies have shown that intranasal administration of OT given in different social situations, is able to enhance the salience of social cues, especially when it is associated with the activation of the dopaminergic system (for review see [65]). For instance, in humans, OT activates Ventral Tegmental Area (VTA) in response to social relevant cues signaling reward or punishment, e.g. friendly or angry faces [66]. Similar studies have also been done in rodents: for example, administration of OT antagonist during mating of individuals that previously acquired strong partner preference seems to reduce their motivation to seek further contact [67]. In teleost fish, the posterior tuberculum (PT) located in the basal diencephalon is the most likely homolog region of mammalian VTA [68]. Perhaps, the occurrence of higher levels of IT at the diencephalon could be a response to the novelty associated to the allopatric cleaners; whether it enhances the dopaminergic activity at the PT remains to be studied.
In conclusion, it appears that in response to unclear/novel and potentially noxious stimuli, nonapeptide’ influence is reported at relevant brain areas: AVT at the level of the optic tectum and the telencephalon (forebrain), and IT at the level of the diencephalon. Moreover, in response to sympatric cleaners, clients showed reduced levels of AVT in the cerebellum, however no differences were found in other areas, between contexts. It is perhaps the overall AVT expression across brain areas (reduction at the cerebellum but maintenance at the other areas) that works as a pre-requisite for mutualistic behaviour to occur. Future complementary research should further test for the direct influence of exogenous administration of AVT and IT on client ability to recognize and interact with cleaners (direct comparison between sympatric and allopatric cleaners). Moreover, it would be worthwhile to extend these tests to juvenile naïve clients, to find out if these would respond similarly to sympatric cleaners as the adult clients do in relation to allopatric cleaners.
Supporting information
In this file are included Tables A, B, C, D, E, F, Fig A. and two methodological descriptions: 1) Subject clients’ brain microdissection procedure and 2) Measurement of gonad 17β-estradiol (E2) for sex identification.
(DOCX)
Acknowledgments
We thank the principal curator of Oceanário de Lisboa (Núria Baylina) and staff for logistical support. Thanks are also due to José R. Paitio for help in the maintenance of the fish facilities during experimental procedures.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was supported by the Portuguese Foundation for Science and Technology-FCT (grant PTDC/MAR/105276/2008 given to MCS). MSC is currently supported by SFRH/BPD/109433/2015. HPLC analyses of AVT and IT, and E2 analyses were partially financed by the National Science Center grant 2012/05/B/NZ4/02410 and by Institute of Oceanology of the Polish Academy of Sciences statutory research task IV.2.2 (given to EK).
References
- 1.Ferguson JN, Young LJ, Insel TR. The neuroendocrine basis of social recognition. Front Neuroendocrinol. 2002; 23: 200–224. doi: 10.1006/frne.2002.0229 [DOI] [PubMed] [Google Scholar]
- 2.Bshary R, Grutter AS. Image scoring and cooperation in a cleaner fish mutualism. Nature. 2006; 441: 975–978. doi: 10.1038/nature04755 [DOI] [PubMed] [Google Scholar]
- 3.Pinto A, Oates J, Grutter AS, Bshary R. Cleaner wrasses Labroides dimidiatus are more cooperative in presence of an audience. Curr Biol. 2011; 21(13): 1140–1144. doi: 10.1016/j.cub.2011.05.021 [DOI] [PubMed] [Google Scholar]
- 4.Bshary R, Bronstein JS. A general scheme to predict partner control mechanisms in pairwise cooperative interactions between unrelated individuals. Ethology 2011; 117: 271–283, doi: 10.1111/J.1439-0310.2011.01882.x [Google Scholar]
- 5.Lehmann L, Keller L. The evolution of cooperation and altruism—a general framework and a classification of models. J Evol Biol. 2006; 5: 1365–1376. doi: 10.1111/j.1420-9101.2006.01119.x [DOI] [PubMed] [Google Scholar]
- 6.McNamara JM, Leimar O. Variation and the response to variation as a basis for successful cooperation. Phil Trans R Soc B. 2010; 365: 2627–2633. doi: 10.1098/rstb.2010.0159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dugatkin LA. Cooperation among animals:an evolutionary perspective 1997. Oxford Universitary Press, New York. [Google Scholar]
- 8.Sachs JL, Mueller UG, Wilcox TP, Bull JJ. The evolution of cooperation. Quart Rev Biol. 2004; 79: 135–160. [DOI] [PubMed] [Google Scholar]
- 9.Hamilton WD. The genetical evolution of social behavior, I & II. J Theor Biol. 1964; 7: 1–52. [DOI] [PubMed] [Google Scholar]
- 10.Herberstein ME, Baldwin HJ, Gaskett AC. Deception Downunder: is Australia a hot-spot for deception? Behav Ecol. 2014; 25: 12–16. doi: 10.1093/beheco/art105 [Google Scholar]
- 11.Bshary R. Machiavellian intelligence in fishes In: Brown C, Laland K, Krause J, editors. Fish cognition and behavior. Oxford, UK: Wiley-Blackwell; 2011. doi: 10.1002/9781444342536.ch13 [Google Scholar]
- 12.Acher R, Chauvet J. The neurohypophysial endocrine regulatory cascade: precursors, mediators, receptors, and effectors. Front Neuroendocrinol. 1995; 16: 237–289. doi: 10.1006/frne.1995.1009 [DOI] [PubMed] [Google Scholar]
- 13.Goodson JL, Bass AH. Social behavior functions and related anatomical characteristics of vasotocin/vasopressin systems in vertebrates. Brain Res Rev. 2001; 35: 246–265. doi: 10.1016/S0165-0173(01)00043-1 [DOI] [PubMed] [Google Scholar]
- 14.Godwin J, Thompson R. Nonapeptides and social behavior in fishes. Horm Behav. 2012; 61(3): 230–238. doi: 10.1016/j.yhbeh.2011.12.016 [DOI] [PubMed] [Google Scholar]
- 15.Saito D, Komatsuda M, Urano A. Functional organization of preoptic vasotocin and isotocin neurons in the brain of rainbow trout: central and neurohypophysial projections of single neurons. Neuroscience. 2004; 124: 973–984. doi: 10.1016/j.neuroscience.2003.12.038 [DOI] [PubMed] [Google Scholar]
- 16.Backström T, Winberg S. Arginine-vasotocin influence on aggressive behavior and dominance in rainbow trout. Physiol Behav. 2009; 96: 470–475. doi: 10.1016/j.physbeh.2008.11.013 [DOI] [PubMed] [Google Scholar]
- 17.Yaeger C, Ros AM, Cross V, Deangelis RS, Stobaugh DJ, Rhodes JS. Blockade of arginine vasotocin signaling reduces aggressive behavior and c-Fos expression in the preoptic area and periventricular nucleus of the posterior tuberculum in male Amphiprion ocellaris. Neuroscience. 2014; 267: 205–218. doi: 10.1016/j.neuroscience.2014.02.045 [DOI] [PubMed] [Google Scholar]
- 18.Santangelo N, Bass AH. New insights into neuroptide modulation of aggression: field studies of arginine vasotocin in a territorial damselfish. Proc Roy Soc B. 2006; 273: 3085–3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Santangelo N, Bass AH. Individual behavioral and neuronal phenotypes for arginine vasotocin mediated courtship and aggression in a territorial teleost. Brain Behav Evol. 2010; 75: 282–291. doi: 10.1159/000316867 [DOI] [PubMed] [Google Scholar]
- 20.Carneiro LA, Oliveira RF, Canário AVM, Grober MS. The effect of arginine vasotocin on courtship behaviour in a blenniid fish with alternative reproductive tactics. Fish Physiol Biochem. 2003; 28: 241–243. [Google Scholar]
- 21.Lema Sc, Nevitt GA. Exogenous vasotocin alters aggression during agonistic exchanges in male Amargosa River pupfish (Cyprinodon nevadensis amargosae). Horm Behav. 2004; 46: 628–637. doi: 10.1016/j.yhbeh.2004.07.003 [DOI] [PubMed] [Google Scholar]
- 22.Semsar K, Kandel FL, Godwin J. Manipulations of the AVT system shift social status and related courtship and aggressive behavior in the bluehead wrasse. Horm Behav. 2001; 40: 21–31. doi: 10.1006/hbeh.2001.1663 [DOI] [PubMed] [Google Scholar]
- 23.Oldfield RG, Hofmann HA. Neuropeptide regulation of social behavior in a monogamous cichlid fish. Physiol Behav 2011; 102: 296–303. doi: 10.1016/j.physbeh.2010.11.022 [DOI] [PubMed] [Google Scholar]
- 24.O'Connell LA, Matthews BJ, Hofmann HA. Isotocin regulates paternal care in a monogamous cichlid fish. Horm Behav. 2012; 61: 725–733. doi: 10.1016/j.yhbeh.2012.03.009 [DOI] [PubMed] [Google Scholar]
- 25.Soares MC, Bshary R, Mendonça R, Grutter AS, Oliveira RF. Neuropeptide modulation of cooperative behaviour: arginine vasotocin decreases prosocial behaviour in cleanerfish. PLoS ONE. 2012; 7: e39583 doi: 10.1371/journal.pone.0039583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cardoso SC, Paitio JR, Oliveira RF, Bshary R, Soares MC. Arginine vasotocin reduces levels of cooperative behaviour in a cleaner fish. Physiol Behav 2015; 139: 314–320. doi: 10.1016/j.physbeh.2014.11.052 [DOI] [PubMed] [Google Scholar]
- 27.Cardoso SC, Bshary R, Mazzei R, Paitio JR, Oliveira RF, Soares MC. Arginine vasotocin modulates associative learning in a mutualistic fish. Behav Ecol Sociobiol. 2015; 69(7): 1173–1181. doi: 10.1007/s00265-015-1931-z [Google Scholar]
- 28.Cardoso SC, Grutter AS, Paula JR, André GI, Messias JP, Gozdowska M, Kulczykowska E, Soares MC. Forebrain neuropeptide regulation of pair association and behavior in cooperating cleaner fish. Physiol Behav. 2015; 145: 1–7. doi: 10.1016/j.physbeh.2015.03.024 [DOI] [PubMed] [Google Scholar]
- 29.Kulczykowska E, Cardoso SC, Gozdowska M, André GI, Paula JR, Slebioda M, Oliveira RF, Soares MC. Brain levels of nonapeptides in four labrid fish species with different levels of mutualistic behavior. Gen Comp Endocrinol. 2015; 222: 99–105. doi: 10.1016/j.ygcen.2015.06.005 [DOI] [PubMed] [Google Scholar]
- 30.Limbaugh C. Cleaning symbiosis. Scient Amer. 1961; 205: 42–49. doi: 10.1038/scientificamerican0861-42 [Google Scholar]
- 31.Feder HM. Cleaning symbiosis in the marine environment In: Henry SM, editor. Symbiosis, New York, Academic Press; 1966. pp. 327–380. [Google Scholar]
- 32.Gorlick DL, Atkins PD, Losey GS. Effect of cleaning by Labroides dimidiatus (Labridae) on an ectoparasite population infecting Pomacentrus vaiuli (Pomacentridae) at Enewetak Atoll. Copeia 1987; 1987(1): 41–45. doi: 10.2307/1446035 [Google Scholar]
- 33.Losey GC, Grutter AS, Rosenquist G, Mahon JL, Zamzow JP. Cleaning symbiosis: a review In: Almada VC, Oliveira RF, Goncalves EJ, editors. Behaviour and conservation of littoral fishes, Instituto Superior de Psicologia Aplicada, Lisbon; 1999. pp. 379–395. [Google Scholar]
- 34.Côté IM. Evolution and ecology of cleaning symbioses in the sea. Oceanogr Mar Biol. 2000; 38: 311–355. [Google Scholar]
- 35.Soares MC, Bshary R, Côté IM. Does cleanerfish service quality depends on client value or choice options? Anim Behav. 2008; 76(1): 123–130. [Google Scholar]
- 36.Bshary R, Côté IM. New perspectives on marine cleaning mutualism In: Magnhagen C, Braithwaite VA, Forsgren E, Kappor B, editors. Fish behaviour. Science Publishers; 2008. pp. 563–592. doi: 10.1201/b10757-20 [Google Scholar]
- 37.Ros AFH, Lusa J, Meyer M, Soares MS, Oliveira RF, Brossard M, Bshary R. Does access to the bluestreak cleaner wrasse Labroides dimidiatus affect indicators of stress and health in resident reef fishes in the Red Sea? Horm Behav. 2011; 59: 151–158. doi: 10.1016/j.yhbeh.2010.11.006 [DOI] [PubMed] [Google Scholar]
- 38.Soares MC, Oliveira RF, Ros AFH, Grutter AS, Bshary R. Tactile stimulation lower stress in fish. Nat Commun 2011; 2: 534 doi: 10.1038/ncomms1547 [DOI] [PubMed] [Google Scholar]
- 39.Cheney KL. Cleaner wrasse mimics inflict higher costs on their models when they are more aggressive towards signal receivers. Biol Lett. 2012; 8:10–12. doi: 10.1098/rsbl.2011.0687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Almeida O, Gozdowska M, Kulczykowska E, Oliveira RF. Brain levels of arginine-vasotocin and isotocin in dominant and subordinate males of a cichlid fish. Horm Behav. 2012; 61: 212–217. doi: 10.1016/j.yhbeh.2011.12.008 [DOI] [PubMed] [Google Scholar]
- 41.Teles MC, Gozdowska M, Kalamarz-Kubiak H, Kulczykowska E, Oliveira RF. Agonistic interactions elicit rapid changes in brain nonapeptide levels in zebrafish. Horm Behav. 2016; 84: 57–63. doi: 10.1016/j.yhbeh.2016.05.020 [DOI] [PubMed] [Google Scholar]
- 42.Teles MC, Dahlbom SJ, Winberg S, Oliveira RF. Social modulation of brain monoamine levels in zebrafish. Behav Brain Res. 2013; 253: 17–24. doi: 10.1016/j.bbr.2013.07.012 [DOI] [PubMed] [Google Scholar]
- 43.Gozdowska M, Ślebioda M, Kulczykowska E. Neuropeptides isotocin and arginine vasotocin in urophysis of three fish species. Fish Physiol Biochem. 2013; 39(4): 863–869. doi: 10.1007/s10695-012-9746-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bshary R, rth M. Cleaner fish Labroides dimidiatus manipulate client reef fish by providing tactile stimulation. Proc R Soc Lond Ser B. 2001; 268: 1495–1501. doi: 10.1098/rspb.2001.1701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bshary R, Grutter AS. Asymmetric cheating opportunities and partner control in a cleaner fish mutualism. Anim Behav. 2002; 63(3): 547–555. doi: 10.1006/anbe.2001.1937 [Google Scholar]
- 46.Soares MC, Bshary R, Cardoso SC, Côté IM. The meaning of jolts by fish clients of cleaning gobies. Ethology. 2008; 114(3): 209–214. doi: 10.1111/j.1439-0310.2007.01471.x [Google Scholar]
- 47.Bshary R, Côté IM.News perspectives on marine cleaning mutualismo In:Fish Behaviour (Magnhagen C, Braithwaite VA, Forsgreen E, Kapoor BG, editors) 2008. Science Publishers, New Hampshire, pp563–592. [Google Scholar]
- 48.Hochberg Y. A Sharper Bonferroni Procedure for Multiple Significance Testing. Biometrika. 1988; 75: 800–803. [Google Scholar]
- 49.R Core Team (2014). R: A language and environment for statistical computing R Foundation for Statistical Computing, Vienna, Austria: Available: http://www.R-project.org/ [Google Scholar]
- 50.Cheney KL, Grutter AS, Blomberg SP, Marshall NJ. Blue and yellow signal cleaning behavior in coral reef fishes. Curr Biol. 2009; 19: 1283–1287. doi: 10.1016/j.cub.2009.06.028 [DOI] [PubMed] [Google Scholar]
- 51.Lettieri L, Streelman JT. Colourful stripes send mixed messages to safe and risky partners in a diffuse cleaning mutualism. J Evol Biol. 2010; 38(23): 2289–2299. doi: 10.1111/j.1420-9101.2010.02098.x [DOI] [PubMed] [Google Scholar]
- 52.Cheney KL, Marshall NJ. Mimicry in coral reef fish: how accurate is this deception in terms of colour and luminance? Behav Ecol 2009; 20(3): 459–468. doi: 10.1093/beheco/arp017 [Google Scholar]
- 53.Cheney KL. The role of avoidance learning in an aggressive mimicry system. Behav Ecol. 2008; 19: 583–588. doi: 10.1093/beheco/arn001 [Google Scholar]
- 54.Salas C, Broglio C, Rodriguez F, López JC, Portavella M, Torres B. Telencephalic ablation in goldfish impairs performance in a spatial constancy problem but not in a cued one. Behav Brain Res. 1996; 79: 193–200. doi: 10.1016/0166-4328(96)00014-9 [DOI] [PubMed] [Google Scholar]
- 55.Salas C, Rodríguez F, Vargas JP, Durán E, Torres B. Spatial learning and memory deficits after telencephalic ablation in goldfish trained in place and turn maze procedures. Behav Neurosci. 1996; 110: 965–980. doi: 10.1037/0735.7044.110.5.965 [DOI] [PubMed] [Google Scholar]
- 56.López JC, Bingman VP, Rodríguez F, Gómez Y, Salas C. Dissociation of place and cue learning by telencephalic ablation in goldfish. Behav Neurosci. 2000; 114(4): 687–699. [DOI] [PubMed] [Google Scholar]
- 57.López JC, Broglio C, Rodríguez F, Thinus-Balnc C, Salas C. Reversal learning deficit in a spatial task but not in a cue one after telencephalic ablation in goldfish. Behav Brain Res. 2000; 109: 91–98. doi: 10.1016/S0166-4328(99)00167-9 [DOI] [PubMed] [Google Scholar]
- 58.Kline RJ, O‘Connell LA, Hofmann HA, Holt GJ, Khan IA. The distribution of an AVT V1a receptor in the brain of a sex changing fish Epinephelus adscensionis. J Chem Neuroanat. 2011; 42: 72–88. doi: 10.1016/j.jchemneu.2011.06.005 [DOI] [PubMed] [Google Scholar]
- 59.Huffman LS, O´Connell LA, Kenkel CD, Kline RJ, Khan IA, Hofmann HA. Distribution of nonapeptide systems in the forebrain of an African cichlid fish, Astatotilapia burtoni. J Chem Neuroanat. 2012; 44: 86–97. doi: 10.1016/j.jchemneu.2012.05.002 [DOI] [PubMed] [Google Scholar]
- 60.Lema SC, Slane MA, Salvesen KE, Godwin J. Variation in gene transcript profiles of two V1a-type arginine vasotocin receptors among sexual phases of bluehead wrasse (Thalassoma bifasciatum). Gen Comp Endocrinol. 2012; 179: 451–464. doi: 10.1016/j.ygcen.2012.10.001 [DOI] [PubMed] [Google Scholar]
- 61.Loveland JL, Fernald RD. Differential activation of vasotocin neurons in contexts that elicit aggression and courtship. Behav Brain Res. 2017; 317: 188–203. doi: 10.1016/j.bbr.2016.09.008 [DOI] [PubMed] [Google Scholar]
- 62.Rodriguez F, Durán E, Gómez A, Ocaña FM, Alvarez E, Jiménez-Moya F, Broglio C, Salas C. Cognitive and emotional functions of the teleost fish cerebellum. Brain Res Bull. 2005; 66: 365–370. doi: 10.1016/j.brainresbull.2004.11.026 [DOI] [PubMed] [Google Scholar]
- 63.Bangasser DA, Waxler DE, Santollo J, Shors TJ. Trace conditioning and the hippocampus: The importance of contiguity. J Neurosci. 2006; 26: 8702–8706. doi: 10.1523/JNEUROSCI.1742-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Soares MC, Oliveira R, Ros AFH., Grutter A, Bshary R. 2011. Tactile stimulation lower stress in fish. Nature Communications. 2: 534 doi: 10.1038/ncomms1547 [DOI] [PubMed] [Google Scholar]
- 65.Modi ME, Young LJ. The oxytocin system in drug discovery for autism: Animal models and novel therapeutic strategies. Horm Behav. 2012; 61: 340–350. doi: 10.1016/j.yhbeh.2011.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Groppe SE, Gossen A, Rademacher L, Hahn A, Westphall L, Gründer G, Spreckelmeyer KN. Oxytocin influences processing of socially relevant cues in the ventral tegmental area of the human brain. Biol Psychiatry. 2012; 74: 172–179. doi: 10.1016/j.biopsych.2012.12.023 [DOI] [PubMed] [Google Scholar]
- 67.Cho MM, DeVries AC, Williams JR, Carter CS. The effects of oxytocin and vasopressin on partner preferences in male and female prairie voles (Microtus ochrogaster). Behav Neurosci. 1999; 113: 1071–1079. doi: 10.1037/0735-7044.113.5.1071 [DOI] [PubMed] [Google Scholar]
- 68.Rink E, Wullimann MF. The teleostean (zebrafish) dopaminergic system ascending to the subpallium (striatum) is located in the basal diencephalon (posterior tuberculum). Brain Res. 2001; 889(1–2): 316–30. doi: 10.1016/S0006-8993(00)03174-7 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
In this file are included Tables A, B, C, D, E, F, Fig A. and two methodological descriptions: 1) Subject clients’ brain microdissection procedure and 2) Measurement of gonad 17β-estradiol (E2) for sex identification.
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.