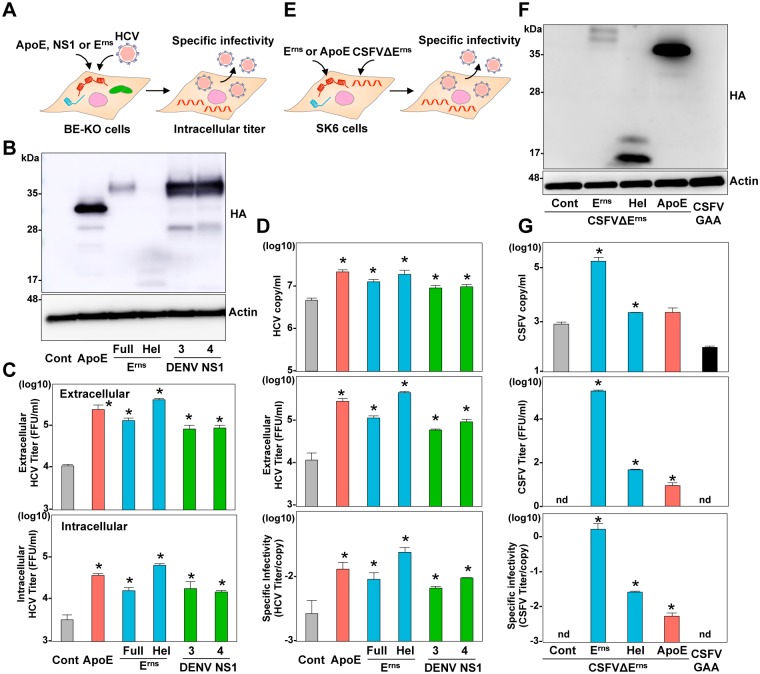
Fig 7. ApoE, Erns, and NS1 participate in the infectious particle formation.
(A) Experimental procedure. (B) Expression of HA-tagged ApoE (HA-ApoE), the full length of HA-Erns, the C-terminal amphipathic α-helix of HA-Erns (Hel), and HA-NS1 from serotype 3 and 4 of DENV was determined by immunoblotting at 48-h post-transduction of lentiviruses into BE-KO cells. (C) Intracellular and extracellular infectious titers were determined at an MOI of 1 by a focus-forming assay. (D) Specific infectivity was calculated as extracellular infectious titers/extracellular HCV RNA copies in BE-KO cells expressing HA-ApoE, HA-Erns, Hel, and HA-NS1 at 72-h post-infection. (E) Experimental procedure. (F) Expression of the HA-tagged Erns, Hel, and ApoE was determined by immunoblotting at 48-h post-transduction of lentiviruses into SK6 cells. (G) Extracellular CSFV RNA and infectious titers were determined at 72-h post-electroporation with CSFVΔErns RNA by qRT-PCR and focus-forming assay, respectively. Specific infectivity was calculated as extracellular infectious titers/extracellular CSFV RNA copies in SK6 cells. In all cases, asterisks indicate significant differences (* p < 0.01) versus the results of the control cells.