Figure 2. RNA exosome-dependent resolution of R-loops during class switch recombination.
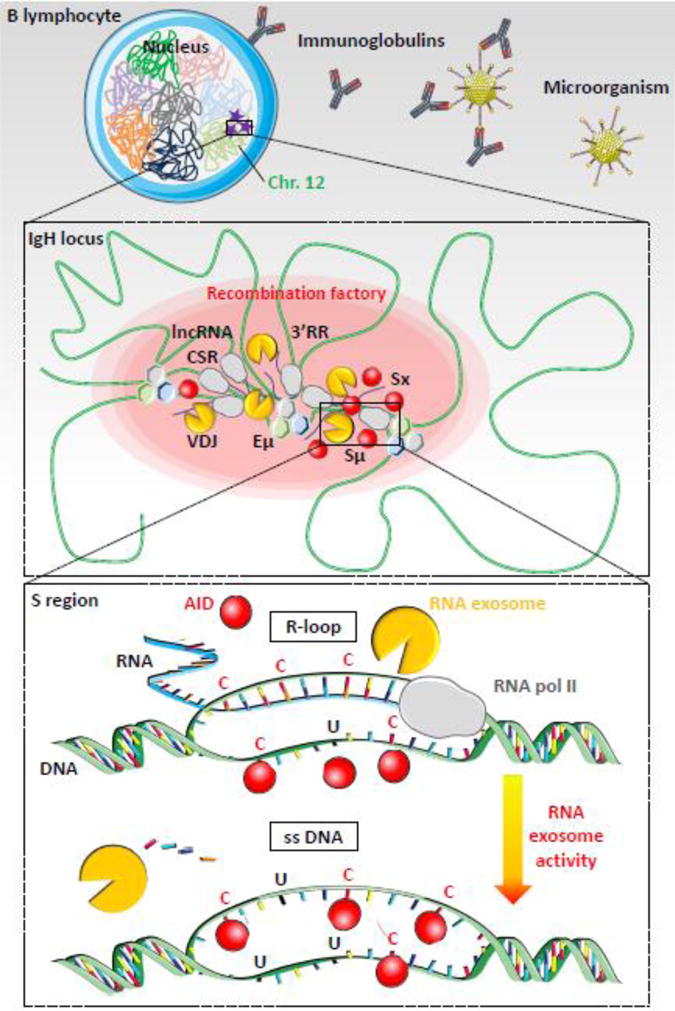
Top. B lymphocyte is shown with nucleus and chromosomes folded in specific chromosomal territories. Mouse IgH loci are located on chromosome 12 (green). AID mutagenic activity is represented as purple stars. Microorganism (for example: an adenovirus) invasion is represented with immunoglobulin production to neutralize pathogen (figure not to scale).
Middle. Chromatin (green) is folded in several loops, induced by transcription factors and architectural proteins, like CTCF, cohesin, mediator or other (hexagons), which bind DNA sequences from distant elements, creating topologically associated domains. Transcription is induced at switch regions and enhancers by RNA polymerase II (grey) producing non-coding RNA (blue lines). RNA exosome (yellow) regulates enhancer activity by degrading eRNA and removes RNA from DNA/RNA hybrids at switch donor (Sμ) and acceptor (Sx) regions. Regulatory elements Eμ, 3′ regulatory region (3′RR), lncRNA-CSR, Iμ and Ix create a hub connecting both switch regions in close conformation and establishing a synapsis for CSR. AID (red) targets switch regions leading to double strand breaks, DNA repair and CSR. These events occur into “recombination factory” (red oval).
Bottom. Switch regions are transcribed by RNA polymerase II (grey). Stalled RNA polII generates RNA/DNA hybrids (R-loops, blue-green hybrid DNA-RNA strand) that prevent DNA accessibility to AID on the transcribed strand. AID indeed targets cytosines (C) on the accessible single strand DNA (ssDNA) of the non-transcribed strand but cannot access the cytosines involved in the R-loops. However, RNA exosome degrades the RNA hybridized to the DNA, generating a new ssDNA and allowing AID accessibility to the second DNA strand. The cytosines are then deaminated to uraciles (U) on both strands and processed by DNA repair mechanisms, leading to double strand breaks in S regions. DNA repair then joins the two S regions to create a new recombined IgH locus utilizing a new constant gene.
