Figure 4.
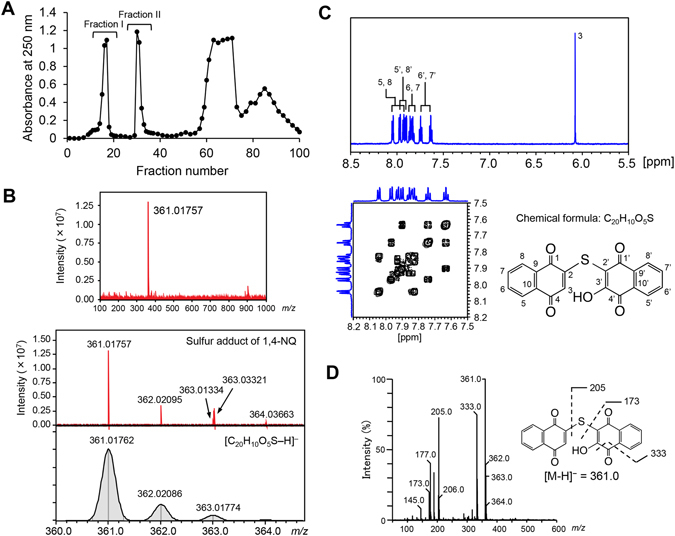
Purification and identification of the sulfur adduct of 1,4-NQ. (A) Separation of sulfur adducts of 1,4-NQ by column chromatography. 1,4-NQ (5 mM) was incubated with Na2S4 (10 mM) for 10 min at room temperature. The resulting solution was applied to an ODS column and eluted with 20% acetonitrile for 40 min followed by 80% acetonitrile for 60 min at a flow rate of 10 mL/min. Each fraction was analyzed by UV absorbance at 250 nm and by UPLC-MS. Fraction II primarily contained m/z 361 in negative ion mode. (B) FT-ICR-MS of the purified sulfur adduct. ESI-MS spectrum of the reaction product with m/z 361 (upper) and comparison of isotope ratios between the product and an elemental composition of C20H10O5S (lower). (C) Magnified views of the 1H NMR (upper) and 1H-1H COSY NMR (lower) spectra of a sulfur adduct of 1,4-NQ with m/z 361. Four doublet proton signals at δ 8.05 (d, J = 3.7 Hz, 1 H), 7.97 (d, J = 3.6 Hz, 1 H), 7.93 (d, J = 3.7 Hz, 1 H) and 7.91 (d, J = 5.8 Hz, 1 H), and four triplet proton signals at δ 7.86 (t, J = 7.4 Hz, 1 H), 7.83 (t, J = 7.4 Hz, 1 H), 7.74 (t, J = 7.5 Hz, 1 H) and 7.63 (t, J = 7.5 Hz, 1 H) were detected. An additional singlet proton signal in the high field at δ 6.07 (s, 1 H) must be attributable to H-3. An aromatic OH group must be located at the C-3′ position, although this OH signal was not detected. The COSY NMR spectrum showed that two triplets at δ 7.74 and δ 7.63 ppm were correlated to one another and to two doublets at δ 7.97 and δ 7.9 ppm, respectively. These signals must be attributable to H-5′, H-6′, H-7′ and H-8′. The other two triplets at δ 7.86 and δ 7.83 ppm were correlated to one another and to two doublets at δ7.93 and δ 8.05 ppm, respectively. These signals must be attributable to H-5, H-6, H-7 and H-8. D: MS spectrum of the sulfur adduct (m/z 361) of 1,4-NQ formed during incubation with Na2S4. The purified sulfur adduct was analyzed by UPLC-MS. Representative data are shown from three independent experiments.
